- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Organotypic Slice Culture of Embryonic Brain Sections
Published: Vol 3, Iss 3, Feb 5, 2013 DOI: 10.21769/BioProtoc.327 Views: 17381

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Cochlear Organ Dissection, Immunostaining, and Confocal Imaging in Mice
Chenyu Chen [...] Dongdong Ren
Jan 20, 2025 3773 Views

Primary Neuronal Culture and Transient Transfection
Shun-Cheng Tseng [...] Eric Hwang
Jan 20, 2025 2728 Views

Isolation and Imaging of Microvessels From Brain Tissue
Josephine K. Buff [...] Sophia M. Shi
Aug 5, 2025 2636 Views
Abstract
This technique will allow using brain slices to study several aspects of cortical development (i.e. neurogenesis), as well as neuronal differentiation (i.e. neuronal migration, axon and dendrite formation) in situ. This protocol is suitable for various embryonic stages (Calderon de Anda et al., 2010; Ge et al., 2010).
Keywords: Neuronal developmentMaterials and Reagents
- Agarose Type VII (low melting) (Sigma-Aldrich, catalog number: A9045-100G )
- Neurobasal (Life Technologies, Gibco®, catalog number: 21103-049 )
- B27 (Life Technologies, InvitrogenTM, catalog number: 17504-04 )
- Glutamine (Life Technologies, Gibco®, catalog number: 25030 )
- Penicillin/streptomycin (Sigma-Aldrich, catalog number: P4458 )
- Horse serum (Life Technologies, InvitrogenTM, catalog number: 16050-122 )
- N2 (Life Technologies, InvitrogenTM, catalog number: 17502-048 )
- 1x PBS
- Krazyglue or similar glue
Equipment
- Vibratome (Leica, model: VT1000 S)
- Millicell, sterilized culture plate insert (EMD Millipore)
- Dissecting tools (Ring forceps, Serrated forceps, Fine scissors)
- Curved spatula
- 30 mm petri dish
Procedure
- Remove the head of electroporated mouse embryos (see “In utero Electroporation of Mouse Embryo Brains” (Ge, 2011) and place it into ice-cold 1x PBS.
- Dissect out the whole brain and place it into ice-cold PBS. Do not let the brain more that 30 min in ice-cold PBS. Faster the brain is processed; better brain slices quality will be obtained.
- Fill a 30 mm petri dish with 3% low-melting agarose prepared in 1x PBS (use enough agarose to cover the brain) pre-warmed at 40 °C in a microwave. Monitor the temperature with a thermometer while steering the agarose solution with it. The brain will be put into the agarose when the temperature drops to about 35 °C-37 °C, try to use fresh 3% low-melting agarose every time you prepare brain slices.
- Take out the brain from the ice-cold PBS with a curved spatula and carefully remove the PBS solution from the tissue with a Kimwipe. Use the Kimwipe as a wick. Critical step to maintain the structure of the brain in culture: Try to remove as much PBS solution as possible from the tissue. Avoid the Kimwipe to adhere to the brain in order to prevent tissue damage. Place the Kimwipe as close as you can to the brain to absorb, as much PBS solution as possible. A “PBS solution-free” brain is better embedded into the agarose solution. Thus, once the brain is sliced the brain sections do not detach from the agarose.
- Place the brain into 3% low-melting agarose (35 °C-37 °C) in a petri dish (olfactory bulb facing down, Figure 1A).
- Put the petri dish on ice and wait until the agarose solidifies. Carefully keep the position of the brain with the spatula meanwhile the agarose solidifies.
- Cut the agarose around the brain and keep the brain in the middle of the agarose block. Try to cut the block with a pyramidal shape in such a way that the base of the brain (cerebellum) is the base of the pyramid and the olfactory bulb faces the apex of the pyramid (Figure 1B). This agarose block configuration will allow a steady position of the block when glued at the vibratome tray’s holder (the base of the agarose pyramid faces the vibratome holder).
- Glue the agarose block containing the brain onto the vibratome tray with Krazyglue or similar glue.
- Add ice-cold 1x PBS into the vibratome tray to cover the agarose block.
- Slices the agarose block. Slice thickness: 240 μm (tissue flatten over time with thinner slices). Vibratome settings: speed 4-5; vibration 6.
- Collect slices with curved spatula carefully to avoid the tissue to detach from the agarose. Transfer the slices into a Millicell culture insert system containing 1 ml medium (Figure C; Neurobasal supplemented with B27, N2, 0.5 mM Glutamine, 1% penicillin/ streptomycin, and 5% horse serum). Put as many slices as you can put on top of the membrane insert (generally 4-5 slices per insert).
- Remove carefully the solution that surrounds the slice with pipette tip. Tissue must be exposed to air.
- Place the culture insert dish with slices into tissue culture incubator (37 °C) for 1-2 h
The slices are ready to use (i.e. Time-lapse imaging). This culture system has been used for 48 h time-lapse imaging (de Anda F., et al., 2010; Ge et al., 2010). Also is possible to use this culture system for pharmacological treatments (de Anda F., et al., 2010; Ge et al., 2010).
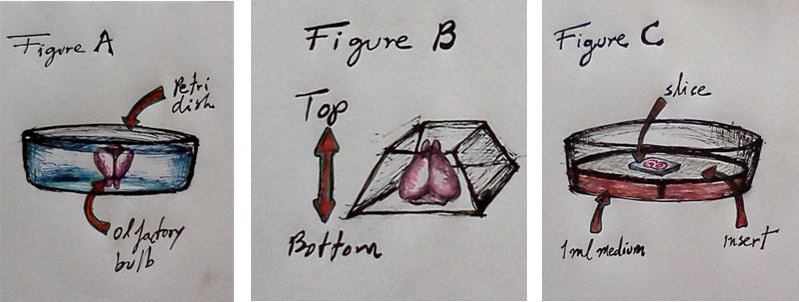
Figure 1. (A) Brain is embedded, with the olfactory bulb facing down, in a preti dish with 3% low-melting agarose. (B) Once the agarose solidifies cut the agarose around the brain with a pyramidal shape in such a way that the base of the brains (side of the cerebellum) is at the base of the pyramid and olfactory bulb faces the apex of the pyramid. (C) Transfer coronal sections from the brain on top of the Millicell culture insert system containing 1 ml medium.
Recipes
- Neurobasal (250 ml)
This media can be stored at 4 °C protected from light for several months50x B27
4.5 ml
200 mM Glutamine
2.5 ml
50x Penicillin/streptomycin
2.5 ml
Horse serum
12.5 ml
100x N2
2.5 ml (it should be added to the medium the day of the experiment; 10 μl/1 ml medium)
Total
24.5 ml/250 ml Neurobasal
Acknowledgments
This protocol was adapted form the previously published paper: Calderon de Anda et al. (2010). C.d.A.F. was supported by a postdoctoral fellowship from the Simons Initiative on Autism and the Brain Infrastructure Grant Program.
References
- Calderon de Anda F., Meletis, K., Ge, X., Rei, D. and Tsai, L. H. (2010). Centrosome motility is essential for initial axon formation in the neocortex. J Neurosci 30(31): 10391-10406.
- Calderon de Anda F., Rosario, A. L., Durak, O., Tran, T., Graff, J., Meletis, K., Rei, D., Soda, T., Madabhushi, R., Ginty, D. D., Kolodkin, A. L. and Tsai, L. H. (2012). Autism spectrum disorder susceptibility gene TAOK2 affects basal dendrite formation in the neocortex. Nat Neurosci 15(7): 1022-1031.
- Ge, X. (2012). In utero Eletroporation of Mouse Embryo Brains. Bio-protocol 2(13): e231.
- Ge, X., Frank, C. L., Calderon de Anda, F. and Tsai, L. H. (2010). Hook3 interacts with PCM1 to regulate pericentriolar material assembly and the timing of neurogenesis. Neuron 65(2): 191-203.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Anda, F. C. D. (2013). Organotypic Slice Culture of Embryonic Brain Sections. Bio-protocol 3(3): e327. DOI: 10.21769/BioProtoc.327.
Category
Developmental Biology > Cell growth and fate > Neuron
Neuroscience > Development > Neuron
Cell Biology > Tissue analysis > Tissue isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









