- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Xenograft Mouse Model of Human Uveal Melanoma
Published: Vol 7, Iss 21, Nov 5, 2017 DOI: 10.21769/BioProtoc.2594 Views: 9370
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Participant-Derived Xenograft Mouse Model to Decode Autologous Mechanisms of HIV Control and Evaluate Immunotherapies
Emma Falling Iversen [...] R. Brad Jones
Apr 5, 2025 2531 Views

Reprogramming White Fat Cells for Adipose Manipulation Transplantation (AMT) Therapy
Kelly An [...] Nadav Ahituv
Aug 5, 2025 2247 Views

Vascularization of Human Pancreatic Islets With Adaptive Endothelial Cells for In Vitro Analysis and In Vivo Transplantation
Ge Li [...] Shahin Rafii
Dec 20, 2025 770 Views
Abstract
Uveal melanoma (UM) is a malignant intraocular tumor in adults. Metastasis develops in almost half of the patients and over 90% of the metastases are in the liver. With the advances in molecular targeting therapy for melanoma, a proper metastasis animal model is of increasing importance for testing the accuracy and effectiveness of systemic therapies. Here, we describe a xenograft model for mimicking human UM liver metastasis by injecting human UM cells into the vitreous cavity in nude mice. The athymic nude mice are immunocompromised and suitable for xenograft tumor growth and metastasis, and intravitreal injection of cells is a quicker and easier operation under a binocular scope, thereby it is simple and effective to test human UM growth and metastasis.
Keywords: Human uveal melanomaBackground
UM is the most common primary intraocular tumor in adults, with an incidence rate varying from 5 to 10 cases per million in the world (Singh et al., 2011). Almost half of the patients develop metastasis within 15 years from initial diagnosis even after treatment or/and removal of primary tumor (Kujala et al., 2003; Weis et al., 2016). In over 4% of the patients, micrometastasis already exists at the time of diagnosis (Finger et al., 2005). The number might be underestimated because of limitations in detection of early UM. Current medical treatments such as enucleation, plaque brachytherapy, proton beam irradiation have been successful in removing or repressing early focal ocular UMs (Dogrusoz et al., 2017). But in general, UM is resistant to the standard chemotherapies and to date no effective systemic treatment is available for metastatic lesions (Goh and Layton, 2016; Carvajal et al., 2017). Although various advances have been made in UM treatment over decades, one-year survival rate after metastasis remains unchanged at 10-15% (Woodman, 2012).
UM arises from uveal melanocytes. Enriched with blood supply and lack of ocular lymph ducts, UM cells mainly spread to distant organs hematogenously. As a result, 95% of UM metastases have a predilection for the liver (Woodman, 2012). The mechanism underlying metastatic transformation of UM is still not clear. Since the liver metastasis is the leading cause of UM-related death (Collaborative Ocular Melanoma Study, 2011), current studies have been focused on the mechanism and molecular targeted prevention of tumor metastasis. Based on the metastatic proclivity, UMs are divided into two categories: class 1 and 2. Class 2 UMs are more inclined to metastasis with primitive stem cell-like gene expression pattern (Harbour and Chao, 2014). The activation of RB/P53, PI3K/AKT and MAPK signaling pathways leads to tumor overgrowth and anti-apoptosis (Coupland et al., 2013; Reichstein, 2017). 85% of primary and metastatic UMs are presented with gain-function mutations in either of two G-protein genes, GNAQ and GNA11 (Shoushtari and Carvajal, 2014). Loss of chromosome 3 or loss-function mutation in BAP1 gene indicates a poor prognosis and metastatic UM (Damato et al., 2011; van Essen et al., 2014). Transcription factors such as ID2, ZEB1 and TWIST1 are involved in UM growth and invasiveness (Chen et al., 2017), expression of PD-1 in UM cells avoids immune destruction by suppressing T cell, facilitating tumor dissemination, and resistance to chemotherapies (Komatsubara and Carvajal, 2017).
Appropriate animal models for UM are critical in understanding molecular mechanisms and evaluating therapeutic effectiveness. Mice are most commonly utilized for tumor models. No spontaneous UM was found in wild-type mice (Stei et al., 2016). Mutation in GNAQ gene could generate choroidal melanoma in mice, but the tumor exclusively metastasizes to the lung (Huang et al., 2015). To date, no genetic animal model mimicking the aforementioned biological and molecular features of human UM has been generated (Stei et al., 2016). By contrast, mouse intraocular xenograft tumor is a widely accepted UM animal model with an effective formation of primary ocular tumors and potential to metastasize to the liver. This article details a protocol to xenograft human UM cells in the vitreous cavity of nude mice, which develops primary tumors in the eye and metastases in the liver in a relatively short period of time.
Materials and Reagents
- 100-mm culture plate (Corning, catalog number: 430293 )
- 15-ml tube (Corning, catalog number: 430052 )
- 30 G blunt needle (BD, catalog number: 305106 )
- Cotton swabs (VWR, catalog number: 89031-270 )
- Glass coverslips (Fisher Scientific, catalog number: 12-550-15 )
- Diapers (VWR, catalog number: 82020-845 )
- Surgeon masks (VWR, catalog number: 10843-149)
Manufacturer: KCWW, Kimberly-Clark, catalog number: 47500 . - Gloves (VWR, catalog number: 82026-426 )
- Head cover (3M, catalog number: S-133S-5 )
- 1-ml syringe (BD, catalog number: 309628 )
- 27 G needle (BD, catalog number: 305109 )
- 200-μl tips (USA Scientific, catalog number: 1111-1700 )
- Athymic nude mice (THE JACKSON LABORATORY, catalog number: 002019 )
- Human UM cell line OCM1 (provided by Dr. Klara Valyi-Nagy in the University of Illinois at Chicago)
- 0.25% trypsin (Mediatech, catalog number: 20-053-Cl )
- Phosphate-buffered saline (PBS) (Mediatech, catalog number: 21-040-CV )
- Mydriatic eye drops are the mixture of Phenylephrine Hydrochloride Ophthalmic Solution (Paragon, NDC 42702-102-15) and Tropicamide Ophthalmic Solution (Bausch & Lomb, NDC 24208-585-64) at the ratio of 1:1
- Hypromellose ophthalmic demulcent solution (Akorn, NDC 17478-064-12)
- GONAK Lubricant (2.5% Hypromellose ophthalmic demulcent solution) (Akorn, NDC 17478-064-12)
- Povidone-Iodine solution (Dynarex, NDC 67777-100-03)
- Ketamine hydrochloride (Hospira, NDC 0409-2053-10)
- Xylazine sterile solution (Akorn, AnaSed®, NDC 59399-110-20)
- Neomycin and polymyxin B sulfates and dexamethasone ophthalmic ointment (Fera, NDC 48102-003-35)
- Meloxicam (Henry Schein, NDC 11695-6925-2)
- 10% neutral buffered formalin (Sigma-Aldrich, catalog number: F5554-4L )
- Ethanol (Sigma-Aldrich, catalog number: 459836-1L )
- Dulbecco’s modified Eagle’s medium (DMEM) (Mediatech, catalog number: 10-013-CV )
- Fetal bovine serum (FBS) (GE Healthcare, HycloneTM, catalog number: SH30070.03HI )
- Penicillin-streptomycin solution (Mediatech, catalog number: 30-002-Cl )
- Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: D5879-500ML )
- Cell culture medium (see Recipes)
- Cell cryopreservation medium (see Recipes)
Equipment
- Cell culture incubator (Thermo Fisher Scientific, Thermo ScientificTM, model: Series 8000 Water-Jacketed , catalog number: 3423)
- Bench top centrifuge (Fisher Scientific, model: accuSpin 24CTM )
- -80 °C freezer (Thermo Fisher Scientific, Thermo ScientificTM, model: TSUTM Series , catalog number: TSU400A-EA)
- 5-μl syringe (Hamilton, catalog number: 87900 )
- 50-ml beaker (VWR, catalog number: 10754-946 )
- Forceps (VWR, catalog number: 82027-404 )
- Scissors (VWR, catalog number: 89259-982 )
- 200-µl pipet (Eppendorf, catalog number: 3123000055 )
- Liquid nitrogen tank (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: CY50945 )
- Surgical microscope (Olympus, model: SZ61/51 )
- Warm pad (Pristech Products, catalog number: 20414 )
- Autoclave (SOMA Technology, model: Steris Amsco Century V116 )
- -20 °C freezer (Kelvinator Commercial, model: KCBM180FQY )
- Sterile hood (Labconco, model: Class II, Type B2 (Total Exhaust) )
Procedure
Note: Athymic nude mice were purchased from the Jackson’s Laboratory, breeding, husbandry, and surgical procedures were in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Central South University or/and by University of Louisville Institutional Animal Care and Use Committee (IACUC).
- Preparation of UM cells
- Dr. Klara Valyi-Nagy in the University of Illinois at Chicago generously provided the human UM cell line OCM1.
- The OCM1 cells are cultured in the medium (Dulbecco’s modified Eagle’s medium [DMEM] with 10% heat-inactivated fetal bovine serum [FBS], 100 U/ml penicillin and 0.1 mg/ml streptomycin, see Recipes) in a 37 °C and 5% CO2 incubator.
- Cells are passaged at 1:3 ratio when confluent by trypsinization with enough 0.25% trypsin solution to cover cell culture plate surface after removal of the culture medium and rinsing of cells twice with phosphate-buffered saline (PBS).
- Trypsin is inactivated by adding the culture medium. Add twice the amount of the medium than trypsin solution used above.
- The trypsinized cells are transferred to a 15-ml tube, centrifuged at 500 x g at 4 °C for 5 min, and resuspended in PBS to make a final concentration of 5 x 104 cells/μl.
- If not used immediately, cells can be resuspended in a cryopreservation medium (the above culture medium with 10% dimethyl sulfoxide, see Recipes) at the concentration of 1 x 106 cells/ml, and then stored in a -80 °C freezer for short-term storage (< one month) or in a liquid nitrogen tank for long-term storage (> one month).
- Dr. Klara Valyi-Nagy in the University of Illinois at Chicago generously provided the human UM cell line OCM1.
- Instrument sterilization
- Prepare a 30 G blunt needle and a 5-µl syringe, place them into a 50-ml beaker with 30 ml of distilled water and boil for 30 min.
- Prepare forceps and scissors, clean the instruments with running water for 5 min, disinfect all the instruments in an autoclave with a standard program (121 °C for 20 min).
- Prepare cotton swabs, 30 G needles, mydriatic eye drops, Povidone-Iodine and erythromycin eye ointment, glass coverslips.
- Place a sterilized diaper underneath a surgical microscope or any binocular scope, establish and maintain a sterile field to minimize possible contamination.
- The operator should wear head cover, mask and sterilized gloves before the operation.
- Prepare a 30 G blunt needle and a 5-µl syringe, place them into a 50-ml beaker with 30 ml of distilled water and boil for 30 min.
- Preparation of anesthesia and positioning of the animal
- Prepare 6-week-old athymic nude mice, by weighing all mice individually to ensure accurate medication dosage for each of them.
- Give an intraperitoneal (IP) anesthesia shot with a 27 G needle and a 1-ml syringe containing a mixture of 100 mg of ketamine and 10 mg of xylazine per kg of the body weight.
- Monitor the effectiveness of the anesthesia until the animal completely falls to sleep prior to following procedure.
- Give a drop of mydriatic eye drop to dilate the pupil.
- Trim eye lashes with a pair of scissors (with some ophthalmic ointment on the blades).
- Give 2 drops of Povidone-Iodine to disinfect the eyes.
- Give a drop of eye lubricant drop to moisture the eyes.
- Place the animal on the diaper and let it lay on one side to facilitate the operation.
- Prepare 6-week-old athymic nude mice, by weighing all mice individually to ensure accurate medication dosage for each of them.
- Intravitreal injection of tumor cell (see Video 1)
- Put a sterilized glass coverslip on the eyeball and focalize the surgical microscope to see the fundus clearly.
- Use a 30 G needle to poke a hole about 1 mm behind the limbus.
- Before injection, use a 200-μl tip to pipet up and down to ensure a single-cell suspension.
- Use Hamilton microliter syringe to inject 2 μl of the above prepared cells into the vitreous, and place the needle posteriorly to avoid touching the lens to avoid cataract formation.
- Press a cotton swab on the incision for 1 min and cover the eye with erythromycin ointment to stop possible bleeding after the injection.
- Inject 2 µl of PBS in the same way into the other eye as a shamed control.Video 1. Resuspending pellet in buffer I or buffer II: Gently rotate bottles in an ice-bucket to resuspend the bacterial pellet in buffer
- Put a sterilized glass coverslip on the eyeball and focalize the surgical microscope to see the fundus clearly.
- Animal care after the operation
- Keep the grafted mice on a warm pad, check their respiration and activity hourly until they revive.
- Keep the mice under specific-pathogen-free condition, maintain the warmth, ventilation and clean the cage as usual.
- Check the wound and add ointment daily (while under anesthesia) for the next 5 days to prevent infection.
- Give 0.1 ml of Meloxicam at 5 mg/ml subcutaneously per 0.25 kg of body weight daily for at least 2 days to reduce wound pain.
- Keep the grafted mice on a warm pad, check their respiration and activity hourly until they revive.
- Tumor growth assessment
- Prepare intraperitoneal anesthesia with 1-ml syringe and 27 G needle containing a mixture of 100 mg of ketamine and 10 mg of xylazine per kg of the body weight.
- Use a drop of mydriatic eye drop to dilate the pupil.
- Put the animal to lay on one side.
- Give a drop of eye lubricant drop and place a glass coverslip on the eyeball.
- Place the animal under the surgical microscope or check the fundus with an indirect ophthalmoscope. The tumor size is estimated in relation to the entire vitreous cavity.
- Prepare intraperitoneal anesthesia with 1-ml syringe and 27 G needle containing a mixture of 100 mg of ketamine and 10 mg of xylazine per kg of the body weight.
- Potential metastasis assessment
- Euthanize the mice 4 weeks later by CO2 inhalation followed by a cervical dislocation.
- Isolate the eye, liver, lung, and kidney for paraffin-embedded sections to check formation of primary tumor and metastasis by histology haemotoxylin and eosin (H&E) staining.
- Euthanize the mice 4 weeks later by CO2 inhalation followed by a cervical dislocation.
- Tumor dissection and histology
- Put all collected tissues in 10% neutral buffered formalin and then in 70% ethanol until further processing for embedding in paraffin.
- Section the tissues at 10-μm for histopathological and immunostaining analyses.
- Put all collected tissues in 10% neutral buffered formalin and then in 70% ethanol until further processing for embedding in paraffin.
Data analysis
- Representative results
The above-described procedure on 16 nude mice resulted in both tumor formation in the eye (87.5%) and the liver (50%). The average time for the tumor to be discovered intraocularly under the ophthalmoscope or outside of the eye was 12.4 days and 23.8 days, respectively (Figures 1A-1C). The mice were euthanized within a week after tumor grew outside of the eyes. Extraocular tumors usually displayed with bleeding and atrophy of the eyes (Figures 1C and 2A), though the retina structure was still maintained relatively intact (Figure 2A). Micrometastasis was detected in the liver tissues around the blood vessels (Figure 2B) (Chen et al., 2017).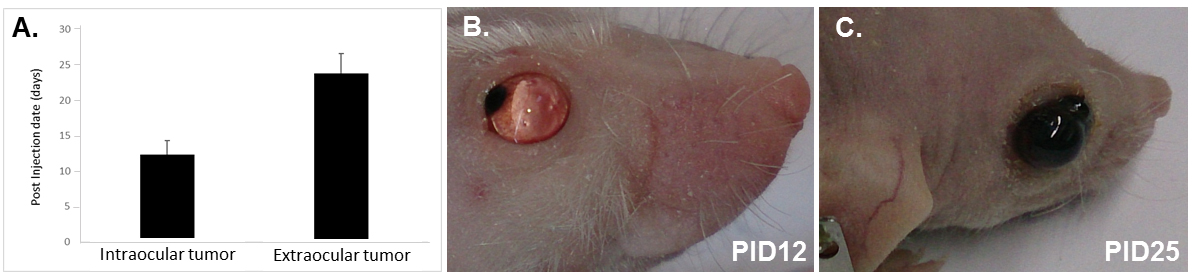
Figure 1. Time-course of tumor growth in nude mice. A. Tumor development inside and outside the eye; B. 12 days after intravitreal injection of tumor cells; C. 25 days after intravitreal injection of tumor cells. PID, post injection day.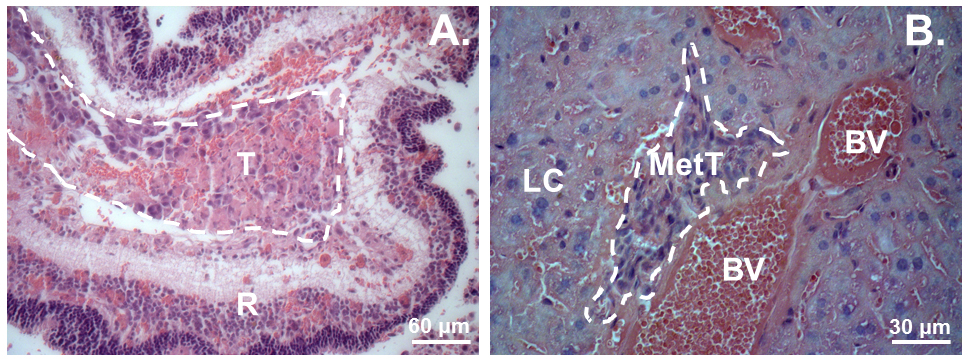
Figure 2. Hematoxylin and eosin (H&E) staining of the tumors in the eye and in the liver. A. A xenograft tumor in the eye; B. Tumor micrometastasis in the liver. T, Tumor. R, Retina. LC, Liver cell. MetT, Metastasized tumor. BV, Blood vessel. - Discussion
- This protocol describes a xenograft tumor model in nude mice to enable study of UM liver metastasis. Nude mice are commonly used in tumor studies for they are immune deficient. Xenograft tumors are generated fast in nude mice comparing to other mice such as C57BL/6, in which we did not observe any intraocular tumor formation by intravitreal injection with OCM1 cells (data not shown). In contrast to genetic and chemical-induced UM models, in which metastatic tumors are rarely generated or the metastases occur solely in other organs such as in the lung, but not in the liver as expected to be, the described xenograft UM model is simple and effective in generating liver metastasis in a relative short period of time. There are other methods using cutaneous melanoma cells for xenograft tumor study; however, the metastasis lesion was detected usually in the lung (Kilian et al., 2016). Human UM cell xenograft tumor model manifests more similarities with human UM development in terms of biological and pathological features. The critical step of this protocol is intravitreal injection. Mice are cost-efficient and easy for care and experimental operations. However, their lenses are extremely large, occupying almost 90% of the vitreous while the vitreous cavity of mice is relatively small, compared to other popular animal models. As a result, the number of implanted UM cells is limited, and the accidental touch of the lens would produce cataract that hampers the observation on follow-up tumor growth. Pointing the needle posteriorly towards the retina can avoid touching the lens. UM xenograft models include anterior chamber or subcutaneous injection of UM tumor cells (de Lange et al., 2012; Kilian et al., 2016), but the vitreous injection of tumor cells may have more effects on the ocular posterior segment, which would mimic the context where most human UMs develop. Although the best option for choroidal melanoma is the sub-RPE injection, the technique is more technically challenging to comprehend with a risk of misinjection into subretinal space or perforating the sclera, and therefore would significantly reduce the success rates.
- Liver micrometastasis was detected in this model when the tumor grew outside of the eye. Nevertheless, no similar micrometastasis was found in the lung, kidney and spleen, which might be due to the short-term observation. Actually, some nude mice died when the tumor grew out of the eye, the reason of death might be ascribed to the bleeding or infection from necrosis of extraocular tumor. The described UM liver metastasis mouse model can be applied for evaluating systemic therapies for UM, which would provide new prospect for preventing metastasis of UM.
- This protocol describes a xenograft tumor model in nude mice to enable study of UM liver metastasis. Nude mice are commonly used in tumor studies for they are immune deficient. Xenograft tumors are generated fast in nude mice comparing to other mice such as C57BL/6, in which we did not observe any intraocular tumor formation by intravitreal injection with OCM1 cells (data not shown). In contrast to genetic and chemical-induced UM models, in which metastatic tumors are rarely generated or the metastases occur solely in other organs such as in the lung, but not in the liver as expected to be, the described xenograft UM model is simple and effective in generating liver metastasis in a relative short period of time. There are other methods using cutaneous melanoma cells for xenograft tumor study; however, the metastasis lesion was detected usually in the lung (Kilian et al., 2016). Human UM cell xenograft tumor model manifests more similarities with human UM development in terms of biological and pathological features. The critical step of this protocol is intravitreal injection. Mice are cost-efficient and easy for care and experimental operations. However, their lenses are extremely large, occupying almost 90% of the vitreous while the vitreous cavity of mice is relatively small, compared to other popular animal models. As a result, the number of implanted UM cells is limited, and the accidental touch of the lens would produce cataract that hampers the observation on follow-up tumor growth. Pointing the needle posteriorly towards the retina can avoid touching the lens. UM xenograft models include anterior chamber or subcutaneous injection of UM tumor cells (de Lange et al., 2012; Kilian et al., 2016), but the vitreous injection of tumor cells may have more effects on the ocular posterior segment, which would mimic the context where most human UMs develop. Although the best option for choroidal melanoma is the sub-RPE injection, the technique is more technically challenging to comprehend with a risk of misinjection into subretinal space or perforating the sclera, and therefore would significantly reduce the success rates.
Notes
- Directly putting live cells, through cryopreservation medium in a vial, into liquid nitrogen results in cell death in large number. Thus, gradually decreasing temperature by leaving the cells first in a -20 °C freezer for one day, and then in a -80 °C freezer for an additional day before storing them in liquid nitrogen significantly preserves cell viability in their long-term cryopreservation.
- Intravitreal injections must be administered under aseptic conditions to avoid ocular infection. Aseptic technique includes the use of topical disinfectant, sterile coverslip, gloves, and surgical draping. All cell culture-related solutions and plastic wares must be sterile, and manipulations must be conducted in a sterile hood.
- Use of xylazine-ketamine mixture for anesthesia may cause acute and reversible cataract that impacts follow-up intravitreal injection of tumor cells. To avoid such a problem, operator may anesthetize mice by IP injection of an alternative drug like avertin at an appropriate dose (i.e., use 350 μl for a 20-g mouse).
- Mouse lens is relatively large while mouse vitreous cavity is extremely small. Accidentally touching the lens by the intravitreal injection needle would cause cataract that hampers follow-up observation of ocular tumor formation. Care therefore must be taken to avoid touching the lens by pointing the needle posteriorly towards the retina, and gently push cells out of the syringe into the vitreous.
Recipes
- Cell culture medium
Dulbecco’s modified Eagle’s medium (DMEM) with 10% heat-inactivated fetal bovine serum (FBS) with 100 U/ml penicillin and 0.1 mg/ml streptomycin - Cell cryopreservation medium
Dulbecco’s modified Eagle’s medium (DMEM) with 10% heat-inactivated fetal bovine serum (FBS) with 10% dimethyl sulfoxide (DMSO)
Acknowledgments
We are grateful to Drs. Klara Valyi-Nagy and Tibor Valyi-Nagy in University of Illinois at Chicago for providing us with OCM1 human UM cell line. This work was supported by the National Natural Science Foundation of China (No. 3087282 and No. 81072221) and the Natural Science Foundation of Hunan Province (14JJ2005) and by the Basic Research Grant of University of Louisville School of Medicine (E0819 to YL), and Research to Prevent Blindness (to DOVS at Louisville), and partially published in Scientific Reports (Chen et al., 2017). The authors have nothing to disclose.
References
- Carvajal, R. D., Schwartz, G. K., Tezel, T., Marr, B., Francis, J. H. and Nathan, P. D. (2017). Metastatic disease from uveal melanoma: treatment options and future prospects. Br J Ophthalmol 101(1): 38-44.
- Chen, Y., Lu, X., Montoya-Durango, D. E., Liu, Y. H., Dean, K. C., Darling, D. S., Kaplan, H. J., Dean, D. C., Gao, L. and Liu, Y. (2017). ZEB1 regulates multiple oncogenic components involved in uveal melanoma progression. Sci Rep 7(1): 45.
- Collaborative Ocular Melanoma Study, G. (2001). Assessment of metastatic disease status at death in 435 patients with large choroidal melanoma in the Collaborative Ocular Melanoma Study (COMS): COMS report no. 15. Arch Ophthalmol 119(5): 670-676.
- Coupland, S. E., Lake, S. L., Zeschnigk, M. and Damato, B. E. (2013). Molecular pathology of uveal melanoma. Eye (Lond) 27(2): 230-242.
- Damato, B., Eleuteri, A., Taktak, A. F. and Coupland, S. E. (2011). Estimating prognosis for survival after treatment of choroidal melanoma. Prog Retin Eye Res 30(5): 285-295.
- de Lange, J., Ly, L. V., Lodder, K., Verlaan-de Vries, M., Teunisse, A. F., Jager, M. J. and Jochemsen, A. G. (2012). Synergistic growth inhibition based on small-molecule p53 activation as treatment for intraocular melanoma. Oncogene 31(9): 1105-1116.
- Dogrusoz, M., Jager, M. J. and Damato, B. (2017). Uveal melanoma treatment and prognostication. Asia Pac J Ophthalmol (Phila) 6(2): 186-196.
- Finger, P. T., Kurli, M., Reddy, S., Tena, L. B. and Pavlick, A. C. (2005). Whole body PET/CT for initial staging of choroidal melanoma. Br J Ophthalmol 89(10): 1270-1274.
- Goh, A. Y. and Layton, C. J. (2016). Evolving systemic targeted therapy strategies in uveal melanoma and implications for ophthalmic management: a review. Clin Exp Ophthalmol 44(6): 509-519.
- Harbour, J. W. and Chao, D. L. (2014). A molecular revolution in uveal melanoma: implications for patient care and targeted therapy. Ophthalmology 121(6): 1281-1288.
- Huang, J. L., Urtatiz, O. and Van Raamsdonk, C. D. (2015). Oncogenic G protein GNAQ induces uveal melanoma and intravasation in mice. Cancer Res 75(16): 3384-3397.
- Kilian, M. M., Loeffler, K. U., Pfarrer, C., Holz, F. G., Kurts, C. and Herwig, M. C. (2016). Intravitreally injected HCmel12 melanoma cells serve as a mouse model of tumor biology of intraocular melanoma. Curr Eye Res 41(1): 121-128.
- Komatsubara, K. M. and Carvajal, R. D. (2017). Immunotherapy for the treatment of uveal melanoma: current status and emerging therapies. Curr Oncol Rep 19(7): 45.
- Kujala, E., Makitie, T. and Kivela, T. (2003). Very long-term prognosis of patients with malignant uveal melanoma. Invest Ophthalmol Vis Sci 44(11): 4651-4659.
- Reichstein, D. (2017). New concepts in the molecular understanding of uveal melanoma. Curr Opin Ophthalmol 28(3): 219-227.
- Singh, A. D., Turell, M. E. and Topham, A. K. (2011). Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology 118(9): 1881-1885.
- Shoushtari, A. N. and Carvajal, R. D. (2014). GNAQ and GNA11 mutations in uveal melanoma. Melanoma Res 24(6): 525-534.
- Stei, M. M., Loeffler, K. U., Holz, F. G. and Herwig, M. C. (2016). Animal models of uveal melanoma: methods, applicability, and limitations. Biomed Res Int 2016: 4521807.
- Weis, E., Salopek, T. G., McKinnon, J. G., Larocque, M. P., Temple-Oberle, C., Cheng, T., McWhae, J., Sloboda, R. and Shea-Budgell, M. (2016). Management of uveal melanoma: a consensus-based provincial clinical practice guideline. Curr Oncol 23(1): e57-64.
- Woodman, S. E. (2012). Metastatic uveal melanoma: biology and emerging treatments. Cancer J 18(2): 148-152.
- van Essen, T. H., van Pelt, S. I., Versluis, M., Bronkhorst, I. H., van Duinen, S. G., Marinkovic, M., Kroes, W. G., Ruivenkamp, C. A., Shukla, S., de Klein, A., Kilic, E., Harbour, J. W., Luyten, G. P., van der Velden, P. A., Verdijk, R. M. and Jager, M. J. (2014). Prognostic parameters in uveal melanoma and their association with BAP1 expression. Br J Ophthalmol 98(12): 1738-1743.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Chen, Y., Liu, X., Gao, L. and Liu, Y. (2017). Xenograft Mouse Model of Human Uveal Melanoma. Bio-protocol 7(21): e2594. DOI: 10.21769/BioProtoc.2594.
Category
Cancer Biology > General technique > Animal models
Cell Biology > Cell Transplantation > Xenograft
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










