- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolation of Fucus serratus Gametes and Cultivation of the Zygotes
Published: Vol 7, Iss 14, Jul 20, 2017 DOI: 10.21769/BioProtoc.2408 Views: 9233
Reviewed by: Rebecca Van AckerAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A β-glucuronidase (GUS) Based Bacterial Competition Assay to Assess Fine Differences in Fitness during Plant Infection
Julien S. Luneau [...] Alice Boulanger
Jul 5, 2022 3222 Views

Transgene-free Genome Editing in Grapevine
Edoardo Bertini [...] Sara Zenoni
Feb 20, 2025 2526 Views

Isolation and Transfection of Protoplasts From Maize Mesophyll Cells
Lauren A. Higa [...] Zhi-Yan Du
Feb 5, 2026 100 Views
Abstract
Zygotes of the Fucale species are a powerful model system to study cell polarization and asymmetrical cell division (Bisgrove and Kropf, 2008). The Fucale species of brown algae grow in the intertidal zone where they reproduce by releasing large female eggs and mobile sperm in the surrounding seawater. The gamete release can be induced from sexually mature fronds in the laboratory and thousands of synchronously developing zygotes are easily obtained. In contrast to other eukaryotic models, such as land plants (Brownlee and Berger, 1995), the embryo is free of maternal tissues and therefore readily amenable to pharmacological approaches. The zygotes are relatively large (up to 100 µm in diameter), facilitating manipulations and imaging studies. During the first hours of zygote development, the alignment of the axis to external cues such as light is labile and can be reversed by light gradients from different directions. A few hours before rhizoid emergence, the alignment of the axis and the polarity are fixed and the cells germinate accordingly. At this stage the zygotes are naturally attached to the substratum through the secretion of cell wall adhesive materials (Kropf et al., 1988; Hervé et al., 2016). The first cell division occurs about 24 h after fertilisation and the early embryo is composed of only two cell types that differ in size, shape and developmental fates (i.e., thallus cells and rhizoid cells) (Bouget et al., 1998). The embryo can be successfully cultivated in the laboratory for a few more days (4 weeks maximum) and has an invariant division pattern during the early stages, which allows cell lineages to be traced histologically.
Keywords: Asymmetric cell divisionBackground
This protocol provides instructions for the isolation of male and female gametes of Fucus spp. used for in vitro fertilisation and discusses how to monitor the development of the resulting zygotes and early embryos. These instructions are for the use of the dioecious species Fucus serratus, but they can be readily adapted to a monoecious Fucale species.
Materials and Reagents
- Razor blade (Gilette)
- Black flat base
Note: This can be a black paper base. - Paper towel (Lucart Professional, catalog number: 864043 )
- 100 μm nylon filter (Sigma-Aldrich, catalog number: NY1H00010 )
- Slides
- Petri dishes (SARSTEDT, catalog number: 82.1472.001 )
- Sexually mature plants of Fucus serratus, males and females
Note: Marine Research Institutes are sometimes equipped with facilities that allow the ordering and sending of such material (see for instance the EMBRC-France website, http://www.embrc-france.fr/en) The algae can be transported in moist tissues for a time preferentially no longer than 2 days. - Approximately 1 L of natural seawater
Note: Alternatively artificial seawater can be prepared or purchased (Sigma-Aldrich, catalog number: S9883 ).
Equipment
- Beaker of approximately 250 ml (Fisher scientific)
- Desk lamp
- Fridge or cold room
- Optical microscope equipped with 20x and/or 40x objectives (Olympus)
- Thermostatic chamber at 13 °C (Pol-Eko Aparatura)
- White light, ideally 90 µmol m-2 sec-1 (Mazda)
- Hermetic black chamber (optional)
Procedure
- Isolation of Fucus serratus gametes and fertilisation
- Collect female and male plants of Fucus serratus on the shore (Figure 1, step A1).
During a certain period of the year (winter time for European coasts), the thallus tips of the Fucale species are swollen into receptacles that contain the fertile conceptacles. On the shore, male plants can be recognized by the orange colour of their conceptacles, due to a carotenoid pigment present in the sperm chromoplasts. The female plants are brown-green in colour (Figure 1B1 and 1B2). Sort female and male plants. To do this, section by hand a conceptacle, mount sections on a microscope slide with a drop of seawater and examine by light microscopy. Typical pictures of female and male conceptacles are shown (Figure 1B3 and 1B4). Isolate all sexually mature fronds in two distinct batches (i.e., male/female). - Wash in seawater to remove epiphytes and remaining sand and wash briefly with tap water (Figure 1, step A2).
- Spread all pieces on paper towel (Figure 1, step A3) making layers, and roll the whole such as for a sushi maki (Figure 1B5 and 1B6). Store at 4 °C in darkness for at least one week.
- To release the female gametes, unroll the paper towels and transfer the mature fronds into a beaker containing seawater (Figure 1, step A4). Incubate one hour in daylight or under artificial light at room temperature. The oogonium, which contains eight individual eggs in F. serratus, will be released first, rapidly rupturing to discharge the eggs. Examine by light microscopy.
Note: The stress induced by the abrupt light and osmotic changes (i.e., switching samples from darkness to daylight and back in seawater) favour the gamete release.
Figure 1. Overview of the key stages in the isolation of Fucus gametes and cultivation of the zygotes. A. The key stages are listed on the left side. B. Representative pictures are shown on the right side for: B1 and B2. Female and male Fucus serratus plants; B3 and B4. Cross sections of their respective gamete-containing conceptacles; B5 and B6. The conditioning of the mature fronds for storage; B7. Typical 4-celled zygotes obtained after 48 h following fertilisation. - At this stage the eggs will rapidly settle at the bottom of the beaker. After 2 h incubation gently remove some seawater with a pipette to concentrate the eggs. Filter the remaining solution containing the eggs using a 100 µm nylon mesh filter to remove aggregates. Place one or two small pieces of male fronds in contact with the newly released eggs (Figure 1, step A5). The male gametes exhibit negative phototropism, therefore placing the beaker in full daylight or under artificial light on a black flat base will favour the sperm movement toward the eggs. The female cells release a sexual pheromone to attract the sperm. Fertilisation will occur within 15 min of mixing sperm with oospheres. After 30 min, examine the release and vitality of the male gametes by light microscopy (Video 1).
Video 1. Fertilisation of a Fucus serratus egg by sperm of the same species. The male gametes are motile and clearly visible as small cells actively swimming in the vicinity of the immotile female egg. Flagella on the male gametes can be detected. The egg is approximately 80 µm large. - After one hour, wash twice by gently removing some of the seawater with a pipette and adding fresh seawater. Filter the remaining solution using a 100 µm nylon mesh filter. Allow the zygotes to grow by placing the beaker at 13 °C with a photoperiod of 12 h of light (90 µmol m2 sec-1) and 12 h of darkness (Figure 1, step A6). The zygotes will naturally adhere to their substratum through the secretion of cell wall adhesive materials.
Note: To ease future examination, the zygotes can be directly cultivated on microscope slides. To do this, concentrate the zygotes to an appropriate density and drop 250 µl of the solution on the slides. The use of polylysine-coated microscope slides will allow the zygotes and early embryos to stay firmly attached.
- Collect female and male plants of Fucus serratus on the shore (Figure 1, step A1).
- Monitoring development of Fucus serratus zygotes and early embryos
- Typical growth conditions are 13 °C with a photoperiod of 12 h of light (90 µmol m2 sec-1) and 12 h of darkness. Different stages of early development can be easily observed by light microscopy. The first cell division occurs around 24 h after fertilisation and divides the embryo in two cell types having distinct shapes and fates (Figure 2).
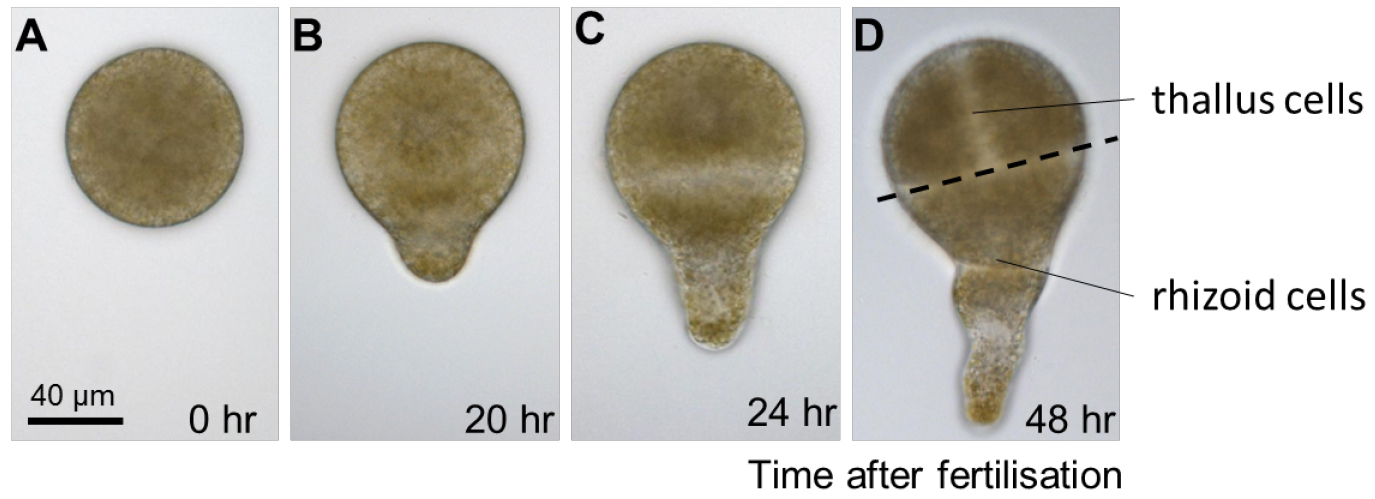
Figure 2. Early development of Fucus serratus zygote and embryo. A. Fucus eggs are spherical in shape with no obvious asymmetries. B. Polarization is first manifested morphologically, around 20 h after fertilisation, when zygotes elongate and produces a bulge on one hemisphere, the rhizoid. C. When the first cell division occurs, about 24 h after fertilisation, it bisects the zygote transversally into two asymmetrical cells with distinct developmental fates: the apical thallus cell and the rhizoid basal cell. D. During the following cell divisions, the rhizoid cell elongates by apical growth whereas the thallus cell expands by diffuse growth. - Regularly change the surrounding seawater to renew nutrients and prevent desiccation. Cultivation in Petri dishes will require the seawater (15-50 ml) to be changed once a week.
- It is possible to control the orientation of the polar axis formation and fixation by applying a light vector. To do this a unilateral light (L1) should be used (a classical light bulb can be used and should not be positioned above the Petri dish but parallel to the bench base). The rhizoid cell will develop in the opposite direction of the light vector L1 (Figure 3A). In normal conditions, the polarization axis is labile during the first 10 h of development and can therefore be redirected with the use of a second unidirectional light gradient (L2), orientated 180° to the first (Figures 3B and 3C). This property allows ready study of the mechanisms controlling polarization by treatment with pharmacological agents. The use of a hermetic black chamber, equipped with a unilateral bulb light, reduces the impact of possible external and uniform light.
- The embryos can be easily grown for one week in the laboratory. Beyond this stage the mature embryos are difficult to maintain in lab culture conditions (4 weeks maximum). Unlike other models of brown algae, such as the Ectocarpus filamentous alga, the life cycle of F. serratus cannot be reproduced in the laboratory.

Figure 3. Control of the orientation of the polar axis formation and fixation. A. Starting just after fertilization (AF), the zygotes are cultivated in a unilateral light direction (L1). The rhizoid cell will develop in the opposite direction of L1. B. Zygotes are cultivated in the unilateral light direction (L1) during the first hours AF. Before axis fixation (approximately 8 h AF), a second unilateral light vector (L2) is applied, oriented 180° from L1. The rhizoid cell develop in the opposite direction of L2. C. If the switch of light directions is applied after axis fixation, the rhizoid cell will develop in the opposite direction of the first light source.
- Typical growth conditions are 13 °C with a photoperiod of 12 h of light (90 µmol m2 sec-1) and 12 h of darkness. Different stages of early development can be easily observed by light microscopy. The first cell division occurs around 24 h after fertilisation and divides the embryo in two cell types having distinct shapes and fates (Figure 2).
Data analysis
Fucus zygotes develop synchronously and the protocol listed above allows the observation of hundreds of individuals at the same time. For data analysis (i.e., morphological results from pharmacological studies) we recommend performing three individual biological replicates. For each replicate a minimum of 30-100 zygotes should be observed. Statistical tests should be applied such as Student’s t-tests (Hervé et al., 2016).
Acknowledgments
A brief description of this protocol has been recently reported in Hervé et al., 2016 and was mainly based on previously described methods (Kropf et al., 1988; Bouget et al., 1998). We acknowledge funding from the Brittany Region (grant ARED_8979 ECTOPAR).
References
- Bisgrove, S. and Kropf, D. L. (2008). Asymmetric cell divisions: zygotes of fucoid algae as a model system. In: Verma, D. and Hong, Z. (Eds.). Cell Division Control in Plants. Springer pp: 323-341.
- Bouget, F. Y., Berger, F. and Brownlee, C. (1998). Position dependent control of cell fate in the Fucus embryo: role of intercellular communication. Development 125(11): 1999-2008.
- Brownlee, C. and Berger, F. (1995). Extracellular matrix and pattern in plant embryos: on the lookout for developmental information. Trends Genet 11(9): 344-348.
- Hervé, C., Siméon, A., Jam, M., Cassin, A., Johnson, K. L., Salmeán, A. A., Willats, W. G., Doblin, M. S., Bacic, A. and Kloareg, B. (2016). Arabinogalactan proteins have deep roots in eukaryotes: identification of genes and epitopes in brown algae and their role in Fucus serratus embryo development. New Phytol 209(4): 1428-1441.
- Kropf, D. L., Kloareg, B. and Quatrano, R. S. (1988). Cell wall is required for fixation of the embryonic axis in Fucus zygotes. Science 239(4836): 187-190.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Siméon, A. and Hervé, C. (2017). Isolation of Fucus serratus Gametes and Cultivation of the Zygotes. Bio-protocol 7(14): e2408. DOI: 10.21769/BioProtoc.2408.
Category
Plant Science > Plant cell biology > Cell isolation
Cell Biology > Cell isolation and culture > Co-culture
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










