- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolation of Ustilago bromivora Strains from Infected Spikelets through Spore Recovery and Germination
Published: Vol 7, Iss 14, Jul 20, 2017 DOI: 10.21769/BioProtoc.2392 Views: 8537
Reviewed by: Arsalan DaudiHiroyuki HiraiAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Rice Ragged Stunt Virus Propagation and Infection on Rice Plants
Chao Zhang [...] Jianguo Wu
Oct 20, 2018 6505 Views

Botrytis cinerea in vivo Inoculation Assays for Early-, Middle- and Late-stage Strawberries
Piao Yang [...] Ye Xia
Oct 20, 2023 2775 Views
Abstract
Ustilago bromivora is a biotrophic smut fungus infecting Brachypodium sp. It is closely related to the barley-infecting smut Ustilago hordei, and related to the well-studied, gall-inducing model pathogen Ustilago maydis. Upon flowering, the spikelets of U. bromivora-infected plants are filled with black fungal spores. While it is possible to directly use this spore material to infect Brachypodium seeds, in many cases it is more useful to isolate individual strains of U. bromivora for a genetically homogenous population. This protocol describes how to collect and germinate the spores of U. bromivora on plate in order to obtain strains derived from a single cell.
Keywords: Brachypodium distachyonBackground
Ustilago maydis infecting maize (Zea mays) has long been established as a model system for studying biotrophic pathogens (Brefort et al., 2009). This has led to many discoveries concerning the nature of biotrophic interactions but has limitations due to the practical difficulties of working with maize in the laboratory. The same is true for the model fungus Ustilago hordei infecting barley (Hordeum vulgare) (Laurie et al., 2012). In contrast to these crop plants, the model grass Brachypodium distachyon has a small genome, undemanding growth conditions and is amenable to genetic manipulation (Draper et al., 2001). B. distachyon has also been used to study non-host resistance to Puccinia striiformis f. sp. tritici due to the genetic complexity of its usual host, wheat (An et al., 2016). Recently, we have described Ustilago bromivora, a smut fungus related to U. maydis, which is able to infect Brachypodium sp. and proposed this as a new model system for studying biotrophic interactions (Rabe et al., 2016).
During infection of Brachypodium sp. by U. bromivora, no visible symptoms can be detected for most of the infection. The only visible symptom of infection occurs during flowering when the plant produces spikelets that are filled with black, fungal spores. These spores can be used to directly infect new seeds but contain genetically disparate fungal strains. For most purposes, a pure culture from a single cell is preferable as it can be cultured axenically, characterized and genetically manipulated before being used to infect further seeds. This protocol describes the process of germinating the U. bromivora spores in vitro. It bears similarities to spore germination protocols of other smut fungi, for example, U. maydis (Heinze, 2009; Nadal et al., 2016), but has been modified to account for differences in the host species and infected organs. Please be aware that U. bromivora shows a mating type bias leading to the survival of only one mating type (mat a) on plate after spore germination (Rabe et al., 2016). This means that it will require additional effort to generate the second mating type or the use of the strain which we have previously isolated.
Materials and Reagents
- Paper bag (HERA, catalog number: 716P50 )
- 1.5 ml microcentrifuge tubes (SARSTEDT, catalog number: 72.690.001 )
- Petri dish (SARSTEDT, catalog number: 82.1473.001 )
- Micro-homogenizer (Carl Roth, catalog number: K994.1 )
- Pipetman Diamond tips, D200 (Gilson, catalog number: F161931 )
- Pipetman Diamond tips, D1000 (Gilson, catalog number: F161671 )
- Glass beads (Sigma-Aldrich, catalog number: 18406 )
- Copper sulfate (CuSO4) (AppliChem, catalog number: 131270 )
- Ampicillin (Carl Roth, catalog number: K029.2 )
- Tetracycline (Duchefa Biochemie, catalog number: T0150 )
- Chloramphenicol (AppliChem, catalog number: A1806 )
- Potato dextrose broth (BD, catalog number: 254920 )
- Agar (BD, catalog number: 214040 )
- PDAmp, Tet, ChlA plates (see Recipes)
Equipment
- Scissors
- Cooled incubator (ST) ST 1 (Pol-Eko Aparatura, catalog number: ST 1 )
- Pipetman P1000 (Gilson, catalog number: F123602 )
- Pipetman P200 (Gilson, catalog number: F123601 )
- Vortex
- HeraeusTM PicoTM 17 Microcentrifuge (Thermo Fisher Scientific, Thermo ScientificTM, model: HeraeusTM PicoTM 17 , catalog number: 75002410)
Procedure
- Harvest the infected spikelets from the plant by cutting them off using a pair of scissors. Figure 1 shows the appearance of the infected spikelets. Spikelets should be stored in a paper bag for ~10 days at 28 °C to dry out. They can then be stored at room temperature until use.

Figure 1. Spikelets of B. distachyon infected by U. bromivora. The black masses of fungal spores are clearly visible in both the hydrated (right) and dehydrated (left) spikelet. The scale bar represents approximately 1 cm. The image background of the spikelets was digitally removed. - Carefully grind the spikelets filled with spore material with a micro-homogenizer in 1.5 ml microcentrifuge tubes to break up the spikelet. The spores are very robust to survive harsh environments and will not be damaged by the grinding.
- Add 500 µl ddH2O and continue to grind softly. It is best to use a rotating motion to grind the spikelet as pushing into the microcentrifuge tube will cause the liquid to splash out. The liquid will turn black as it is ground, indicating that the spores have been released and are properly suspended.
- Incubate the ground spores in ddH2O for 1 h at room temperature to allow faster-germinating, contaminating, fungal spores to germinate. This will leave them more susceptible to the sterilisation treatment and reduce overall contamination levels.
- Add 500 µl 3% CuSO4 and mix the solution either by pipetting up and down or by using a vortex. This will kill most, if not all, of the contaminating spores that have germinated but not the U. bromivora spores which can take up to 17 h to germinate.
Note: CuSO4 is a heavy metal and should be handled and discarded according to its MSDS. - Incubate the spore/CuSO4 mixture for 15 min at room temperature.
- Centrifuge the spore mixture at 1,200 x g for 5 min. This will cause the spores to pellet. Carefully pour out the liquid and re-suspend the spores in 1 ml ddH2O.
- Repeat step 7 three times but, on the third time, instead of re-suspending the spores in ddH2O, proceed to step 9.
- Re-suspend the spores in 300 µl ddH2O with antibiotics (ampicillin, tetracycline and chloramphenicol). These will kill any non-fungal cells that might have survived the CuSO4 treatment.
- Make a dilution series of the fungal spore suspension (100-10-4) in ddH2O with the three antibiotics.
- Plate 100 µl of each dilution on a PDAmp, Tet, ChlA plate (see Recipes), spread using glass beads and incubate at 21 °C for several days to obtain colonies.
- As U. bromivora spores contain tetrads, the original colonies should be singled out on PD plates (with or without antibiotics) to obtain colonies derived from a single cell.
Notes
While we have provided information on the specific equipment and reagents used, we have no reason to believe that it is essential to use them exactly. The equivalent equipment or reagents from other manufacturers should be just as suitable.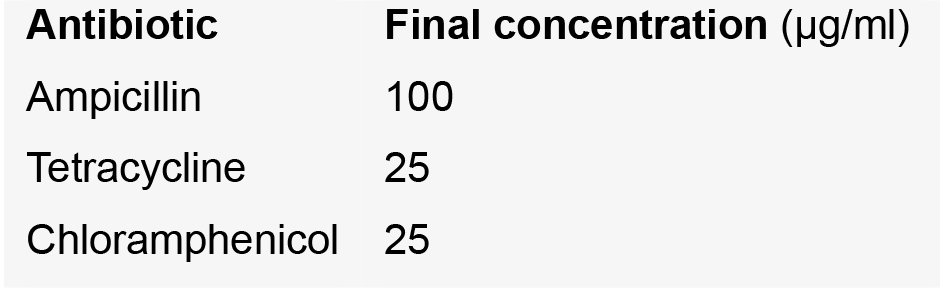
Recipes
- PDAmp, Tet, ChlA plates
2.4% (weight/volume) potato dextrose broth
2.0% (weight/volume) agar
Note: Measure out the appropriate amount of potato dextrose broth and agar for the intended volume. Dissolve them in ddH2O then autoclave the mixture at 121 °C for 15 min. Once it has cooled to approximately 50 °C, add ampicillin, tetracycline and chloramphenicol to their final concentrations. Pour the mixture into Petri dishes (20 ml per 9 cm Petri dish) and wait ~20 min for it to set.
Acknowledgments
This protocol was developed in the Djamei laboratory and has had aspects contributed and/or changed by multiple members of the Djamei group. Due to this, the people listed as authors are not the only ones who have been involved in developing the protocol (for this see Rabe et al., 2016) but are merely the ones who have prepared it for publication. The research leading to these results has received funding from the European Research Council under the European Union’s Seventh Framework Programme (FP7/2007-2013)/ERC grant agreement No. [EUP0012 ‘Effectomics’], the Austrian Science Fund (FWF): [P27429-B22, P27818-B22, I 3033-B22], and the Austrian Academy of Science (OEAW).
References
- An, T., Cai, Y., Zhao, S., Zhou, J., Song, B., Bux, H. and Qi, X. (2016). Brachypodium distachyon T-DNA insertion lines: a model pathosystem to study nonhost resistance to wheat stripe rust. Sci Rep 6: 25510.
- Brefort, T., Doehlemann, G., Mendoza-Mendoza, A., Reissmann, S., Djamei, A. and Kahmann, R. (2009). Ustilago maydis as a pathogen. Annu Rev Phytopathol 47: 423-445.
- Draper, J., Mur, L. A., Jenkins, G., Ghosh-Biswas, G. C., Bablak, P., Hasterok, R. and Routledge, A. P. (2001). Brachypodium distachyon. A new model system for functional genomics in grasses. Plant Physiol 127(4): 1539-1555.
- Heinze, B. (2009). Comparative analysis of the maize smut fungi Ustilago maydis and Sporisorium reilianum. Philipps-Universitat Marburg.
- Laurie, J. D., Ali, S., Linning, R., Mannhaupt, G., Wong, P., Guldener, U., Munsterkotter, M., Moore, R., Kahmann, R., Bakkeren, G. and Schirawski, J. (2012). Genome comparison of barley and maize smut fungi reveals targeted loss of RNA silencing components and species-specific presence of transposable elements. Plant Cell 24(5): 1733-1745.
- Nadal, M., Takach, J., Andrews, D., and Gold, S. (2016). Mating and progeny isolation in the corn smut fungus Ustilago maydis. Bio Protoc: e1793.
- Rabe, F., Bosch, J., Stirnberg, A., Guse, T., Bauer, L., Seitner, D., Rabanal, F. A., Czedik-Eysenberg, A., Uhse, S., Bindics, J., Genenncher, B., Navarrete, F., Kellner, R., Ekker, H., Kumlehn, J., Vogel, J. P., Gordon, S. P., Marcel, T. C., Munsterkotter, M., Walter, M. C., Sieber, C. M., Mannhaupt, G., Guldener, U., Kahmann, R. and Djamei, A. (2016). A complete toolset for the study of Ustilago bromivora and Brachypodium sp. as a fungal-temperate grass pathosystem. Elife 5.
Article Information
Copyright
Bosch and Djamei. This article is distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Bosch, J. and Djamei, A. (2017). Isolation of Ustilago bromivora Strains from Infected Spikelets through Spore Recovery and Germination. Bio-protocol 7(14): e2392. DOI: 10.21769/BioProtoc.2392.
- Rabe, F., Bosch, J., Stirnberg, A., Guse, T., Bauer, L., Seitner, D., Rabanal, F. A., Czedik-Eysenberg, A., Uhse, S., Bindics, J., Genenncher, B., Navarrete, F., Kellner, R., Ekker, H., Kumlehn, J., Vogel, J. P., Gordon, S. P., Marcel, T. C., Munsterkotter, M., Walter, M. C., Sieber, C. M., Mannhaupt, G., Guldener, U., Kahmann, R. and Djamei, A. (2016). A complete toolset for the study of Ustilago bromivora and Brachypodium sp. as a fungal-temperate grass pathosystem. Elife 5.
Category
Plant Science > Plant immunity > Disease bioassay
Cell Biology > Tissue analysis > Tissue staining
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










