- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Plasma Membrane Preparation from Lilium davidii and Oryza sativa Mature and Germinated Pollen
Published: Vol 7, Iss 10, May 20, 2017 DOI: 10.21769/BioProtoc.2297 Views: 9171
Reviewed by: Samik BhattacharyaCindy AstAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Isolation and Biophysical Characterization of Extracellular Vesicles From Hairy Root Cultures
Marisa Conte [...] Alfredo Ambrosone
Mar 5, 2025 2218 Views

Rapid Miniprep of Intact Chloroplasts from Arabidopsis thaliana Leaves
Brenda A. Carranza-Correa [...] Manuel Gutiérrez-Aguilar
May 20, 2025 2645 Views

Isolation and Transfection of Protoplasts From Maize Mesophyll Cells
Lauren A. Higa [...] Zhi-Yan Du
Feb 5, 2026 99 Views
Abstract
Pollen germination is an excellent process to study cell polarity establishment. During this process, the tip-growing pollen tube will start elongating. The plasma membrane as the selectively permeable barrier that separates the inner and outer cell environment plays crucial roles in this process. This protocol described an efficient aqueous polymer two-phase system followed by alkaline solution washing to prepare Lilium davidii or Oryza sativa plasma membrane with high purity.
Keywords: Mature pollen grainsBackground
Pollen plasma membrane contains various proteins that are vital for pollen tube growth and fertilization, such as receptor-like kinases (Wang et al., 2016) and ion channels (Hamilton et al., 2015). Isolating pure plasma membrane (PM) is the premise for the comprehensive PM proteome analysis. There are mainly four methods for PM preparation: differential centrifugation, density gradient centrifugation, preparative free-flow electrophoresis and the aqueous polymer two-phase system. Normally, differential centrifugation is often combined with density gradient centrifugation together to separate the subcellular components according to their size, shape and density. This technique is rapid, but due to the organelle density’s overlap, the resultant PM yield and purity are low (Schindler and Nothwang, 2006). Both free-flow electrophoresis and the aqueous polymer two-phase system separate membrane vesicles according to their surface properties. These two methods can enrich PM pure enough for proteomic analysis (Alexandersson et al., 2007). However, the instrument for the free-flow electrophoresis is complicated to operate (Sandelius et al., 1986). On the contrast, the aqueous polymer two-phase system can be performed easily and rapidly with centrifugation, making this method more convenient for PM preparation. PM enriched by the aqueous polymer two-phase system present in the form of vesicles which contain some cytoplasm contaminations (Alexandersson et al., 2008). Treatment with alkaline solution (100 mM Na2CO3, pH 11.5) can open these vesicles into sheets to release the contaminations (Fujiki et al., 1982).
Materials and Reagents
- 1,000 μl pipette tips (Corning, Axygen®, catalog number: T-1000-B )
- 20 x 10 cm envelope
- 50 ml tube (Corning, catalog number: 430829 )
- 60 x 15 mm Petri dish (Corning, catalog number: 430196 )
- 150 x 25 mm Petri dish (Corning, catalog number: 430599 )
- Gauze
- 10 ml tube (Biosharp, catalog number: BS-100-M )
- 100 μm cell strainer (Corning, Falcon®, catalog number: 352360 )
- 2 ml microtube (SARSTEDT, catalog number: 72.694.005 )
- 4 ml ultracentrifuge tube (Beckman Coulter, catalog number: 355603 )
- 26.3 ml ultracentrifuge tube (Beckman Coulter, catalog number: 355654 )
- Lily mature pollen grains harvest according to Han et al. (2010)
- Rice mature pollen grains harvest according to Dai et al. (2007)
- Boric acid (H3BO3) (Sigma-Aldrich, catalog number: B9645 )
- Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P9541 )
- Calcium chloride (CaCl2) (Sigma-Aldrich, catalog number: C2661 )
Note: This product has been discontinued. - Sucrose (Sigma-Aldrich, catalog number: S7903 )
- Calcium nitrate tetrahydrate, Ca(NO3)2·4H2O (Sigma-Aldrich, catalog number: C1396 )
- Thiamine hydrochloride (VB1) (Sigma-Aldrich, catalog number: T4625 )
- Poly (ethylene glycol), average Mn 4,000 (PEG 4000) (Sigma-Aldrich, catalog number: 81240 )
- 3-(N-Morpholino)propanesulfonic acid (MOPS) (Sigma-Aldrich, catalog number: M1254 )
- Ethylenediaminetetraacetic acid (EDTA) (Sigma-Aldrich, catalog number: E6758 )
- DL-Dithiothreitol (DTT) (Sigma-Aldrich, catalog number: D0632 )
- Phenylmethanesulfonyl fluoride (PMSF) (Sigma-Aldrich, catalog number: P7626 )
- cOmplete, EDTA-free protease inhibitor cocktail tablets (Roche Diagnostics, catalog number: 04693132001 )
- L-ascorbic acid (VC) (Sigma-Aldrich, catalog number: A7506 )
- Poly (vinylpolypyrrolidone) (PVPP) (Sigma-Aldrich, catalog number: 77627 )
- Potassium phosphate tribasic (K3PO4) (Sigma-Aldrich, catalog number: P5629 )
- Polyethylene glycol, average mol wt 3,350 (PEG 3350) (Sigma-Aldrich, catalog number: P4338 )
- Dextran T-500 (Pharmacia, catalog number: 17-0320-01 )
- Sodium carbonate (Na2CO3) (Sigma-Aldrich, catalog number: S7795 )
- Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A1933 )
- Lily pollen germination medium (see Recipes)
- Rice pollen germination medium (see Recipes)
- Homogenate buffer (see Recipes)
- Plasma membrane isolation buffer (see Recipes)
- Aqueous polymer two-phase system (see Recipes)
- Dilution buffer (see Recipes)
- Washing buffer (see Recipes)
Equipment
- Pipette (Gilson, model: P1000N )
- Vortex
- Balance
- Centrifuge (Beckman Coulter, model: J2-HS )
- Homogenizer (MP Biomedicals, model: FastPrep®-24 )
- Ultra-centrifuge (Beckman Coulter, model: OptimalTM L-80XP )
- Microscope with 5x and 10x objective (Carl Zeiss, model: Axio Imager 1 )
Software
- ZEN lite software (2012, blue edition)
Procedure
- Mature pollen grain collection
- Use 20 x 10 cm envelope to collect the mature pollen grains (MPGs) at noon (11:00 AM-1:00 PM) when the anthers are dehiscing.
- Transfer the MPGs to 50 ml tubes.
- Use the MPGs immediately or add allochroic silica gels to them and then store at -80 °C.
- Use 20 x 10 cm envelope to collect the mature pollen grains (MPGs) at noon (11:00 AM-1:00 PM) when the anthers are dehiscing.
- In vitro pollen germination
The initiation procedures of in vitro germination are different for lily and rice pollen. For lily pollen, there should be a pretreatment to wash out the thick lipid coat, while the thin-walled rice pollen does not need this wash.- Lilium davidii pollen germination
Note: This procedure was modified from Ren et al. (1998) and Prado et al. (2004).- Transfer the -80 °C stored MPGs to -20 °C in the dark for 2 weeks before the in vitro germination.
- Put 0.5 g MPGs into a dark, humid environment at 4 °C for 10 h.
Note: The dark, humid environment is custom made as follows: Put MPGs in a Petri dish (60 x 15 mm) (Figure 1A), covered with gauze (Figure 1B), and then placed them in a larger Petri dish (150 x 25 mm) with 50 ml ddH2O (Figure 1C). This arrangement only permitted gauze contact with the ddH2O, do not let the MPGs contact the ddH2O directly.
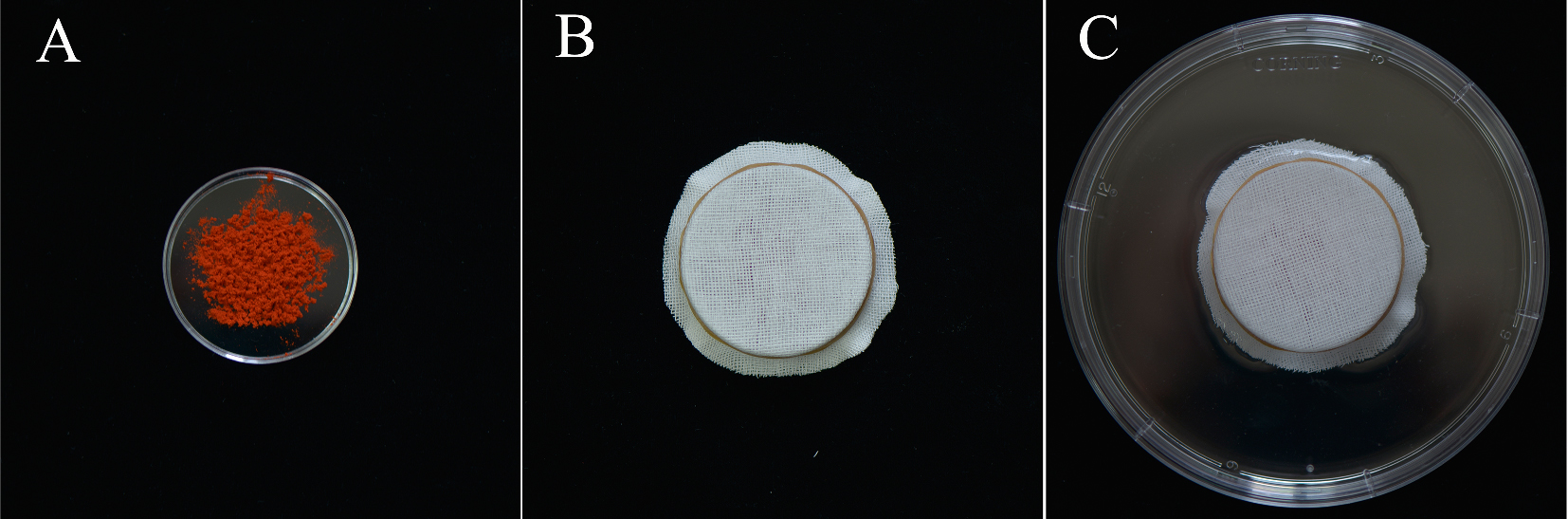
Figure 1. Set up of the humid environment. A. Put 0.5 g lily MPGs in a 60 x 15 mm Petri dish; B. Cover the Petri dish with four layers of gauzes; C. Put the set of B into a 150 x 25 mm Petri dish with 50 ml ddH2O.- Wash the MPGs twice with 5 ml germination medium in a 10 ml tube and discard the flow-through after centrifuging at room temperature at 4,500 x g for 5 min.
- Put the washed MPGs (Figure 2A) into 100 ml germination medium in a Petri dish (60 x 15 mm), and then incubate them in the dark at 28 °C for 2 h with gentle shaking at 75 rpm/min.
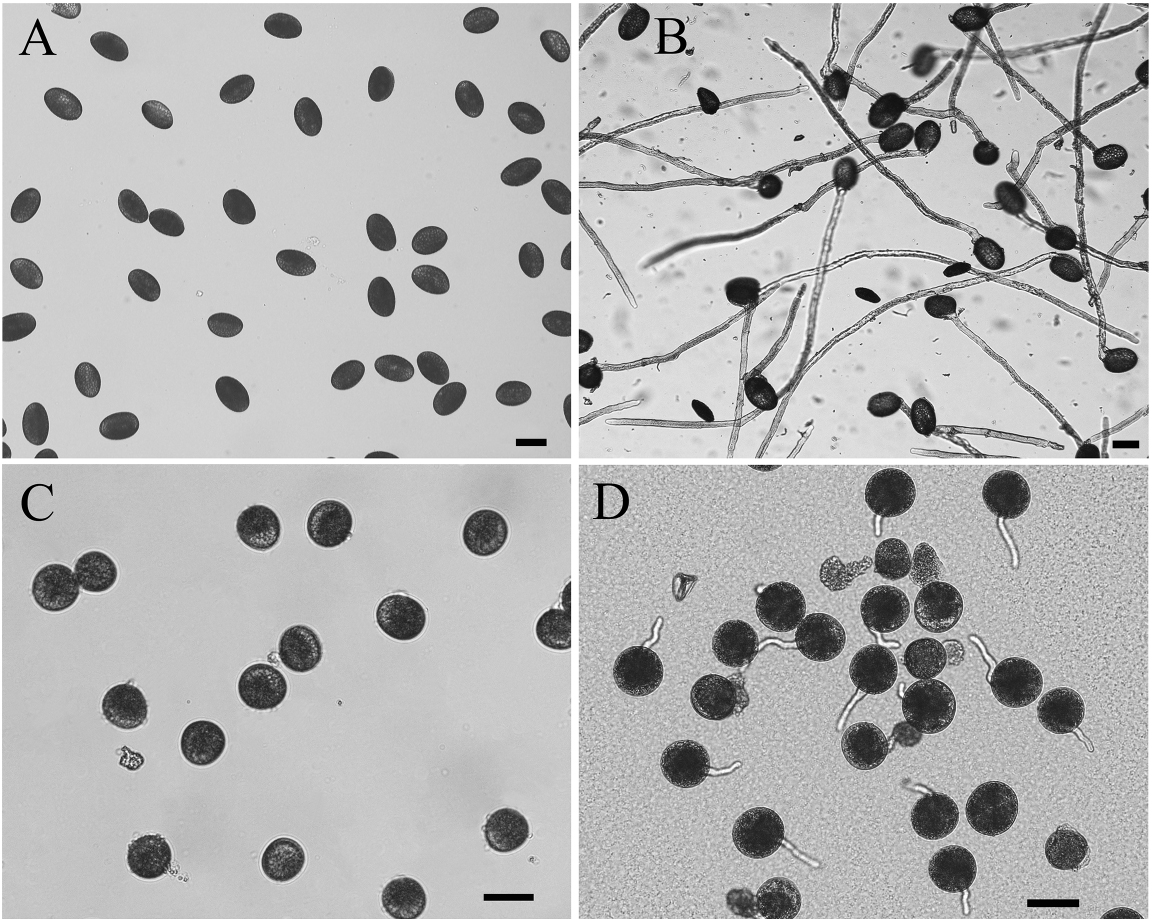
Figure 2. Morphology of Lilium davidii and Oryza sativa MPGs and GPGs. A. Lilium davidii MPGs; B. Lilium davidii MPGs in vitro germinated for 2 h; C. Oryza sativa MPGs; D. Oryza sativa MPGs in vitro germinated for 15 min. Bars = 50 μm. - Use microscope with 5x objective to observe the germination rate, make sure the germination rate is more than 90% and the pollen tube is approximately 500 μm long (Figure 2B).
- Collect the germinated pollen grains (GPGs) by using 100 μm cell strainers, discard the flow-through that contains the ungerminated MPGs.
- Use the GPGs immediately or store them (transfer the mass retained by the strainer to 50 ml tubes, do not add any solution) at -80 °C.
- Transfer the -80 °C stored MPGs to -20 °C in the dark for 2 weeks before the in vitro germination.
- Oryza sativa pollen germination
Note: This procedure was modified from Dai et al. (2007).- Put 0.5 g freshly collected MPGs (Figure 2C) immediately into 100 ml germination medium in a Petri dish and culture them at room temperature with gentle shaking for about 15 min.
Note: Rice MPGs must be freshly collected, if not, they will lose the activity for germination. - Use microscope with 10x objective for observation to get synchronously germinated pollen tubes (Figure 2D).
Note: If pollen germination is not synchronized, or the germination rate is less than 80%, the material should not be used for GPGs PM proteomic analysis. - Collect the GPGs through centrifugation at 4 °C, 1,000 x g, 5 min.
- Use the GPGs immediately or store at -80 °C.
- Put 0.5 g freshly collected MPGs (Figure 2C) immediately into 100 ml germination medium in a Petri dish and culture them at room temperature with gentle shaking for about 15 min.
- Lilium davidii pollen germination
- Microsomal vesicles isolation
- Use 6 ml homogenate buffer to suspend 0.5 g pollens (MPGs or GPGs) in a 50 ml beaker, and then transfer the suspension to three 2 ml microtubes with equal volume.
Note: Lily MPGs should be washed as step B1c before starting step C1. - Homogenize the pollens in 2 ml microtubes by using the FastPrep®-24 at the speed of 6.5 m/sec for 120 sec.
Note: For rice pollens, using the speed of 6.5 m/sec for 30 sec is enough. - Centrifuge the suspension at 4 °C, 1,500 x g for 5 min to remove cell debris, and then transfer the supernatants to new tubes.
- Centrifuge the resulting supernatants at 4 °C, 12,000 x g for 20 min to remove mitochondria, then transfer the supernatants to new tubes.
- Centrifuge the resulting supernatants at 4 °C, 31,000 x g for 15 min to remove other organelle contaminants, then transfer the supernatants to ultracentrifuge tubes.
- Ultracentrifuge the resulting supernatants at 4 °C, 100,000 x g for 1 h, the resulting pellets are the total microsomal vesicles (MSVs).
- Use 6 ml homogenate buffer to suspend 0.5 g pollens (MPGs or GPGs) in a 50 ml beaker, and then transfer the suspension to three 2 ml microtubes with equal volume.
- Plasma membrane enrichment
Aqueous polymer two-phase system is an effective tool to enrich plasma membrane.
The following protocol was designed according to the principle of Schindler and Nothwang (2006).- Suspend the MSVs in 1.2 ml plasma membrane (PM) isolation buffer.
- Put 1.0 g suspended MSVs into the 8.0 g aqueous polymer two-phase system, mix by vortex and then centrifuge at 4 °C, 4,200 x g for 30 min.
Note: The aqueous polymer two-phase system were prepared by using the electronic balance, because the concentrations of the components PEG 3350 and Dextran T-500 were mass ratios (see Recipes). - Transfer 3.0 ml of the top layer into another 8.0 g aqueous polymer two-phase system, mix and then centrifuge at 4 °C, 4,200 x g for 30 min.
- Repeat step D3 once.
- Transfer 3.0 ml of the top layer to an ultracentrifuge tube, dilute it 3-5 folds with dilution buffer, centrifuge at 4 °C, 200,000 x g for 1 h. The resulting pellets are PM vesicles.
- Suspend the resulting pellets with 50 μl dilution buffer for immediate use or store this suspension at -80 °C.
- Suspend the MSVs in 1.2 ml plasma membrane (PM) isolation buffer.
- Plasma membrane purification
Alkaline solution can change the PM vesicles into PM sheets to remove the imbedded cytoplasm. The following protocol was mainly according to Fujiki et al. (1982).- Dilute the PM vesicles with the washing buffer to protein concentration at 0.02~1.00 μg/μl.
- Keep in an ice-bath for 30 min with occasionally vortexing at every 10 min.
- Centrifuge at 4 °C, 50,000 x g for 1 h. Discharge the supernatants and the resulting pellets are the purified PM.
- Wash the purified PM pellets gently with cold ddH2O, don’t suspend the pellets, and then discard the ddH2O.
Note: Usually, starting with 0.5 g pollen can get about 20 μg purified PM proteins. Their purity can be tested through Western blot by using antibodies for PM-specific P-type H+-ATPase and some organelle specific proteins, such as nuclear protein histone H1. - Use the purified PM immediately or store the pellets at -80 °C.
- Dilute the PM vesicles with the washing buffer to protein concentration at 0.02~1.00 μg/μl.
Data analysis
Digital images of lily and rice germinated pollens were obtained using an upright light microscope (Axio Imager 1, Carl Zeiss, Germany) and ZEN lite software (2012, blue edition).
Notes
Don’t over homogenize the pollens. Too much cell debris will reduce the capacity of aqueous polymer two-phase system for plasma membrane purification. The homogenization will be fine when 80% pollens (8 out of 10 pollens) are broken observed under microscope.
Recipes
Note: Use MilliQ water to prepare the following solutions, do not need to autoclave or sterilize by filtration.
- Lily pollen germination medium
1.6 mM H3BO3
1.0 mM KCl
500 μM CaCl2
15% (w/v) sucrose - Rice pollen germination medium
40 mg/L H3BO3
3 mM Ca(NO3)2·4H2O
3 mg/L VB1
10% (w/v) PEG4000
250 mM sucrose - Homogenate buffer
250 mM sucrose
Note: For lily pollen, use 15% (w/v) sucrose.
50 mM MOPS, pH 7.8
1 mM EDTA
1 mM DTT
1 mM PMSF
1x protease inhibitor cocktail
Note: For lily pollen, add 5 mM ascorbic acid, 0.6% (w/v) PVPP, 1% (m/v) PMSF. - Plasma membrane isolation buffer
250 mM sucrose
5 mM potassium phosphate, pH 7.8
1 mM DTT
1 mM PMSF - Aqueous polymer two-phase system
6.5% (w/w) PEG3350
6.5% (w/w) Dextran T-500
250 mM sucrose
5 mM KCl
5 mM potassium phosphate, pH 7.8
1 mM DTT
Note: For lily, use 6.3% (w/w) PEG3350 and 6.3% (w/w) Dextran T-500. - Dilution buffer
250 mM sucrose
50 mM MOPS/KOH, pH 7.8
1 mM DTT
1 mM PMSF - Washing buffer
100 mM sodium carbonate, pH 11.5
Acknowledgments
This protocol was modified from Han et al. (2010) and Yang and Wang (2017). This work was supported by the Chinese Ministry of Science and Technology (grant No. 2013CB945101) and the China Postdoctoral Science Foundation (grant No. 2016M591284).
References
- Alexandersson, E., Gustavsson, N., Bernfur, K., Karlsson, A., Kjellbom, P. and Larsson, C. (2008). Purification and proteomic analysis of plant plasma membranes. Methods Mol Biol 432: 161-173.
- Alexandersson, E., Gustavsson, N., Bernfur, K., Kjellbom, P. and Larsson, C. (2007). Plasma membrane proteomics. In: Šamaj, J. and Thelen, J. J. (Eds). Plant Proteomics. Springer Berlin Heidelberg, pp: 186-206.
- Dai, S., Chen, T., Chong, K., Xue, Y., Liu, S. and Wang, T. (2007). Proteomics identification of differentially expressed proteins associated with pollen germination and tube growth reveals characteristics of germinated Oryza sativa pollen. Mol Cell Proteomics 6(2): 207-230.
- Fujiki, Y., Hubbard, A. L., Fowler, S. and Lazarow, P. B. (1982). Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J cell biol 93(1): 97-102.
- Hamilton, E. S., Jensen, G. S., Maksaev, G., Katims, A., Sherp, A. M. and Haswell, E. S. (2015). Mechanosensitive channel MSL8 regulates osmotic forces during pollen hydration and germination. Science 350(6259): 438-441.
- Han, B., Chen, S., Dai, S., Yang, N. and Wang, T. (2010). Isobaric tags for relative and absolute quantification- based comparative proteomics reveals the features of plasma membrane-associated proteomes of pollen grains and pollen tubes from Lilium davidii. J Integr Plant Biol 52(12): 1043-1058.
- Prado, A. M., Porterfield, D. M. and Feijo, J. A. (2004). Nitric oxide is involved in growth regulation and re-orientation of pollen tubes. Development 131(11): 2707-2714.
- Ren, D. T., Han, S. C., Yan, L. F. and Yen, L. F. (1998). Actin and myosin during pollen germination. Chin Sci Bull 43: 690-694.
- Sandelius, A. S., Penel, C., Auderset, G., Brightman, A., Millard, M. and Morre, D. J. (1986). Isolation of highly purified fractions of plasma membrane and tonoplast from the same homogenate of soybean hypocotyls by free-flow electrophoresis. Plant Physiol 81(1): 177-185.
- Schindler, J. and Nothwang, H. G. (2006). Aqueous polymer two-phase systems: effective tools for plasma membrane proteomics. Proteomics 6(20): 5409-5417.
- Wang, T., Liang, L., Xue, Y., Jia, P. F., Chen, W., Zhang, M. X., Wang, Y. C., Li, H. J. and Yang, W. C. (2016). A receptor heteromer mediates the male perception of female attractants in plants. Nature 531(7593): 241-244.
- Yang, N. and Wang, T. (2017). Comparative proteomic analysis reveals a dynamic pollen plasma membrane protein map and the membrane landscape of receptor-like kinases and transporters important for pollen tube growth and interaction with pistils in rice. BMC Plant Biol 17(1): 2.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Yang, N., Han, B., Liu, L., Yang, H. and Wang, T. (2017). Plasma Membrane Preparation from Lilium davidii and Oryza sativa Mature and Germinated Pollen. Bio-protocol 7(10): e2297. DOI: 10.21769/BioProtoc.2297.
Category
Plant Science > Plant cell biology > Organelle isolation
Plant Science > Plant cell biology > Cell isolation
Cell Biology > Organelle isolation > Membrane
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link














