- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolation and Cultivation of Primary Brain Endothelial Cells from Adult Mice
Published: Vol 7, Iss 10, May 20, 2017 DOI: 10.21769/BioProtoc.2294 Views: 15836
Reviewed by: Oneil G. BhalalaAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Identification and Sorting of Adipose Inflammatory and Metabolically Activated Macrophages in Diet-Induced Obesity
Dan Wu [...] Weidong Wang
Oct 20, 2025 2243 Views

Selective Enrichment and Identification of Cerebrospinal Fluid-Contacting Neurons In Vitro via PKD2L1 Promoter-Driven Lentiviral System
Wei Tan [...] Qing Li
Nov 20, 2025 1343 Views

Revisiting Primary Microglia Isolation Protocol: An Improved Method for Microglia Extraction
Jianwei Li [...] Guohui Lu
Dec 5, 2025 1475 Views
Abstract
Brain endothelial cells are the major building block of the blood-brain barrier. To study the role of brain endothelial cells in vitro, the isolation of primary cells is of critical value. Here, we describe a protocol in which vessel fragments are isolated from adult mice. After density centrifugation and mild digestion of the fragments, outgrowing endothelial cells are selected by puromycin treatment and grown to confluence within one week.
Keywords: Primary cultureBackground
The blood-brain barrier protects the brain from uncontrolled entry of cells and substances. This is mainly achieved by brain endothelial cells that form a barrier composed of tight and adherens junctions to restrict paracellular transport.
This protocol was developed to overcome the limited availability of mouse brain endothelial cell lines that maintain their key characteristics, e.g., the expression of sufficient amounts of tight junction proteins such as occludin, ZO-1 or claudin-5 to induce a high transendothelial resistance.
In addition, the isolation of brain endothelial cells from genetically modified mice allows investigating of gene-specific functions in vitro.
Using this method, we previously complemented in vivo studies demonstrating the importance of NF-κB signaling in brain endothelial cells for maintaining normal blood-brain barrier function (Ridder et al., 2015).
Materials and Reagents
- Materials
- Multiwell plate (cell culture grade) (Greiner Bio One International, catalog number: 662160 )
- Cellulose chromatography paper (sterilize at 180 °C) (Whatman, catalog number: 3030-931 )
- 50 ml centrifuge tubes (cell culture grade) (Greiner Bio One International, catalog number: 210261 )
- 10 ml disposable pipette (Greiner Bio One International, catalog number: 607160 )
- Mice (C57BL/6, age 6 weeks up to 1 year from Charles River, Germany or the in-house breeding facility)
- Ice
- Multiwell plate (cell culture grade) (Greiner Bio One International, catalog number: 662160 )
- ReagentsReagentsManufacturerBrandCatalog numberPreparationAliquotsStorageStock concentrationWorking concentrationDilution1Hydrochloric acid (HCl) 1 N (sterile filtered)Carl Roth1 LRT0.05 N1 ml HCl 1 N + 19 ml H2O, sterile2Dulbecco’s PBS (DPBS)Biowest500 ml4 °C1x1xundiluted370% EtOH (denatured)Th. GeyerRT4IsofluraneBaxterRT5Collagenase/dispase
(sterile filtered)Roche Diagnostics500 mg/5 ml H2O, sterile200 µl-20 °C100 mg/ml1 mg/ml100 µl collagenase/dispase in 10 ml medium6DNase IRoche Diagnostics100 mg/10 ml H2O, sterile100 µl-20 °C10 mg/ml4 µg/ml40 µl DNase I in 10 ml medium7Nα-Tosyl-L-lysine chloromethyl ketone hydrochloride (TLCK)Sigma-Aldrich14.7 mg/10 ml H2O, sterile200 µl-20°C1.47 mg/ml diluted to 14.7 µg/ml0.147 µg/ml100 µl TLCK in 10 ml medium8PuromycinSigma-Aldrich2.5 mg/10 ml H2O, sterile500µl-20°C0.25 mg/ml8 µg/ml32 µl puromycin in 1 ml medium9Trypsin-EDTA 0.25%Thermo Fisher ScientificGibcoTM100 ml-20°C1x1xundiluted104% paraformaldehydeMerck Millipore4% paraformaldehyde in DPBS-20°C11CD31BDBD Pharmingen5573551:50012α-smooth muscle Actin (α-SMA)Acris Antibodies13Iba1Wako Pure Chemical Industries14Glial Fibrillary Acidic Protein (GFAP)EMD Millipore15Zona occludens-1
(ZO-1)Thermo Fisher ScientificInvitrogen16VE-CadherinSanta Cruz Biotechnology17Claudin-5 (Cldn-5)Thermo Fisher ScientificInvitrogen18Occludin (Ocln)Sigma-Aldrich19Mouse collagen, type IVCorning890 mg/ml 0.05 N HCl100 µl-80 °C890 mg/ml50 µg/ml56 µl collagen IV + 944 µl 0.05 N HCl20Dextran MW 60,000-90,000Alfa Aesar1 kgRT18%5.4 g dextran in 30 ml DPBS21Penicillin/streptomycin (100x)Biochrom100 ml1 ml-20 °C100x1x100 µl pen/strep in 10 ml medium/dextran22L-glutamineThermo Fisher ScientificGibcoTM100 ml1 ml-20 °C200 mM (100x)2 mM (1x)100 µl L-glutamine in 10 ml medium23DMEM-F12 w/o glutamineThermo Fisher ScientificGibcoTM500 ml4 °C1x1xundiluted24DMEM w/o glucoseThermo Fisher ScientificGibcoTM500 ml4 °C1x1xundiluted25Plasma-derived bovine serum (PDS)First Link500 ml10 ml-20 °C100%20%10 ml PDS in 50 ml medium26Antibiotic/antimycotic (100x)Thermo Fisher ScientificGibcoTM100 ml1 ml-20 °C100x1x100 µl AA in 10 ml medium/dextran27Heparin-sodiumRatiopharmPZN 0030298431 ampule4 °C5,000 I.E./ml750 I.E./50 ml150 µl heparin in 50 ml medium28Endothelial Cell Growth Supplement (ECGS)Sigma-Aldrich15 mg/5 ml DPBS500 µl-20 °C3 mg/ml30 µg/ml500 µl ECGS in 50 ml medium2918% dextran solution (see Recipes)30Working medium (see Recipes)31Digestion medium (see Recipes)32Full medium (see Recipes)Note: Mouse collagen, type IV: Defrost stock vial slowly on ice at 4 °C overnight. Vortex thoroughly. Aliquot and store at -80 °C. The collagen concentration varies from lot to lot. Therefore, the amount of HCl added has to be adjusted for every new lot.
Equipment
- Refrigerator (4 °C)
- Shaker
- Sterile beakers 100-150 ml (sterilize at 180 °C)
- Laminar flow work bench
- Dounce tissue grinder, 15 ml, autoclave (Sigma-Aldrich, catalog number: D9938 )
- Scalpel
- Tweezers (sterilize at 180 °C)
- Centrifuge (Hettich Lab Technology, model: UNIVERSAL 320 R ),
- Fixed-angle rotor (Hettich Lab Technology, catalog number: 1620A )
- Big scissors (sterilize at 180 °C)
- Small scissors (sterilize at 180 °C)
- Pipette or vacuum pump
- Water bath
- Microwave oven
Procedure
- Preparations (Day 1)–Coating of wells with collagen
- Defrost one collagen aliquot for 2 wells of a 6-well plate or an according volume for other well sizes (see Table 1) slowly (2-3 h) on ice in a refrigerator (4 °C)
If necessary, defrosting aliquots in the refrigerator without ice is possible.
Table 1. Volume adjustment according to well size
- Dilute collagen to 50 µg/ml with 0.05 N HCl.
Note: 0.05 N HCl aliquots can be stored at -20 °C. - Vortex thoroughly. At least 10 sec until small bubbles form (turn the tube to ensure that the viscous collagen stock solution does not continuously stick to the bottom).
- Coat wells evenly with collagen solution (volume see Table 1).
- Put the plate on a shaker, 1 h at room temperature, 25 rpm.
- Move the plate to 4 °C for storage (overnight possible).
Note: It is also possible to coat the plates on the day of endothelial cell preparation.
- Defrost one collagen aliquot for 2 wells of a 6-well plate or an according volume for other well sizes (see Table 1) slowly (2-3 h) on ice in a refrigerator (4 °C)
- Isolation (Day 2)
- Preparations:
- Ice
- Sterilization of instruments
- Approx. 50 ml DPBS in a 150 ml sterile beaker on ice (for each sample).
- Disinfectant (70% EtOH) in a sterile beaker on ice (approx. 50 ml in 150 ml sterile beaker)
- Switch on laminar flow and prepare cellulose chromatography paper, tissue grinder, scalpel, tweezers and 50 ml centrifuge tubes (one for each sample).
- Precool centrifuge to 4 °C.
- Ice
- Anesthetize mice according to your local animal regulations. We use an overdose of isoflurane, which leads to breathing arrest within one minute. Decapitate the mouse with a big scissor and dip the head in ethanol (on ice). Remove the brain swiftly (Figure 1A) and store it in DPBS on ice. Repeat for all brains.
- Cut off cerebellum and olfactory bulb.
- Remove meninges by rolling the brains on cellulose chromatography paper using blunt tweezers.
- Cut cerebrum in 2 to 4 pieces and put the pieces in 5 ml working medium (4 °C). Repeat for all brains.
- Transfer brains with 5 ml working medium (4 °C) into a tissue grinder (Figure 1E) and homogenize (30 strokes with pistil A, 25 strokes with pistil B, Figure 1F). Use a maximum of 10 brains in one tissue grinder.
- Transfer homogenate into a 50 ml centrifuge tube. Rinse tissue grinder with 5 ml working medium (4 °C) and add to the homogenate (10 ml altogether).
- Centrifuge homogenate at 1,350 x g, 5 min, 4 °C. Remove supernatant carefully using a pipette or vacuum pump.
- Resuspend the pellet in 15 ml dextran solution and vortex extensively (2 min). The result is a white, cloudy, homogenous suspension (Figure 1G).
- Centrifuge at 6,080 x g, 10 min, 4 °C. In the meantime, supplement digestion medium with 100 µl collagenase/dispase, 40 µl DNase I and 100 µl TLCK each per 10 ml digestion medium. Pre-warm digestion medium to 37 °C.
- After centrifugation, remove the fluffy myelin layer (top, black arrows in Figures 1H and 1I) and the dextran as completely as possible. Use a 10 ml disposable pipette. Remove the filter of the pipette first if necessary.
- Resuspend the pellet (white arrows in Figures 1H and 1I) in 10 ml digestion medium (37 °C).
- Digest the tissue for 1 h 15 min in a 37 °C water bath (shake from time to time for 2 to 3 sec–approx. every 15 min).
- Centrifuge cell suspension at 1,350 x g, 5 min, room temperature. In the meantime, get the pre-coated plate from the refrigerator, fill sterile DPBS (10 ml per sample) in a centrifuge tube and heat it to 37 °C. Optionally, supplement full medium with puromycin and pre-warm to 37 °C (see step B2q).
- Remove digestion medium.
- Resuspend pellet in 10 ml warm DPBS.
- Centrifuge at 1,350 x g, 5 min, room temperature. In the meantime, remove collagen from the coated wells and wash twice with DPBS. DPBS from the second wash is left in the wells until cells are ready for seeding.
- Remove DPBS and resuspend the pellet in full medium. 2.5 ml full medium per well for a 6-well plate. Use 4-6 brains per culture plate.
- Mix cell suspension carefully before seeding to ensure even distribution.
- Add puromycin as indicated in Table 2. (Can be added directly to the full medium, see step B2l).
Table 2. Culture volume according to well size
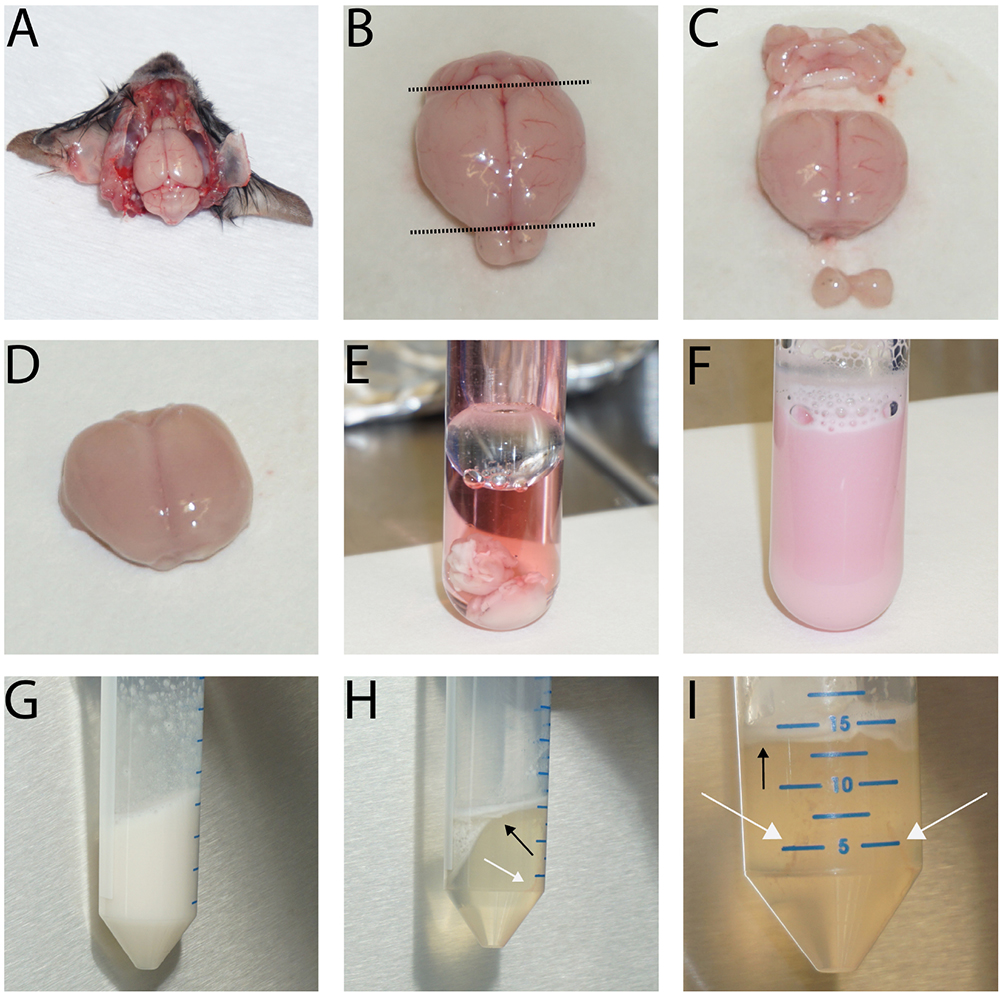
Figure 1. Typical images for the preparation of the brain, removal of meninges, homogenization and subsequent dextran gradient centrifugation. The images depict the first steps of the isolation procedure showing the brain in situ after removal of the skullcap (A), before (B. cut planes indicated by dashed line) and after removal of cerebellum, olfactory bulb (C) and the meninges (D). Then, collect the brains in a Dounce tissue grinder (E), homogenize them (F), and centrifuge the tissue homogenate. Next, resuspend the cells in the dextran solution and vortex extensively (G). Following the centrifugation, the resulting myelin layer is at the top while the vessel fragments collect around the edge of the tube bottom (H + I, black arrows: myelin layer, white arrows: pellet location). The size of the vessel fragment pellet depends on the number of brains used. In E-I, two brains were used for the preparation.
- Cut off cerebellum and olfactory bulb.
- Day 3
- Wash cells twice with DPBS.
- Change full medium.
- Add puromycin (alternatively, puromycin can be added in advance to the full medium).
- Wash cells twice with DPBS.
- Day 4
- Change full medium.
Note: No puromycin needed anymore.
- Preparations:
- Cultivation
- Change medium 1-2 times per week, first time approx. 4-6 days after isolation.
- Split the culture 1:2 (or 1:3) if the cells are confluent. Use trypsin 5-10 min and inactivate with full medium.
- Plate cells and change medium the next day.
- Change medium 1-2 times per week, first time approx. 4-6 days after isolation.
- Purity of the cell culture (Figures 2 and 3)
Endothelial cells (CD31+) > 95%
Pericytes (α-SMA+) < 5%
No astrocytes (GFAP+), microglia (Iba1+), neurons (NeuN+)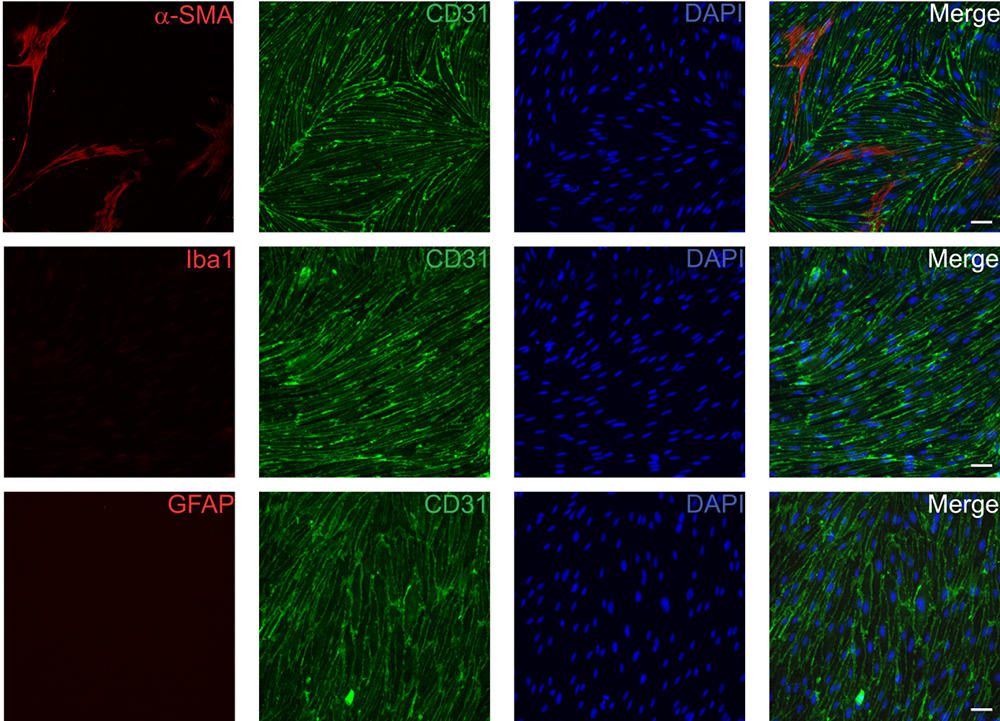
Figure 2. Representative immunofluorescence images of primary mouse brain endothelial cells. Cells were fixed with 4% paraformaldehyde 14 days after isolation and subsequently stained for CD31 (BD, 1:500) as an endothelial cell specific marker in combination with α-SMA (pericytes and smooth muscle cells, Acris, 1:200, upper row), Iba1 (microglia, Wako Pure Chemical Industries, 1:100, middle row) and GFAP (astrocytes, Millipore, 1:400, lower row). Scale bars represent 50 µm.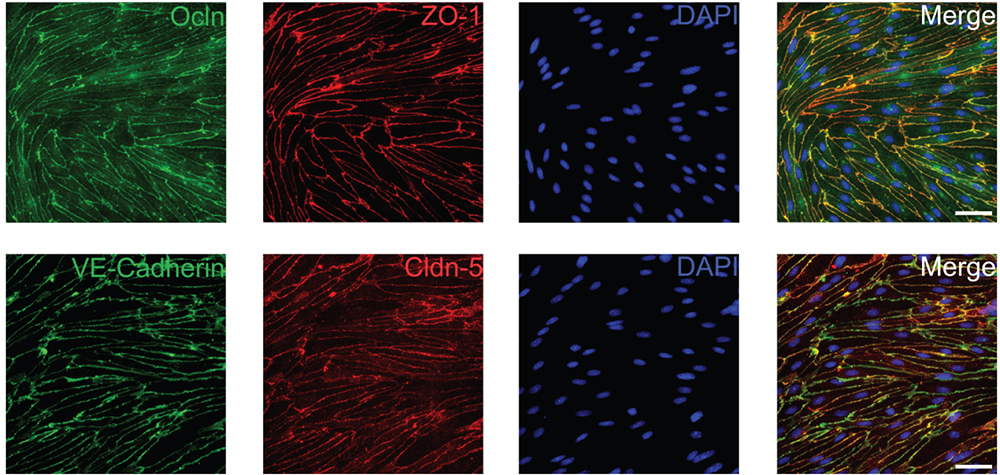
Figure 3. Primary mouse brain endothelial cells maintain expression of tight and adherens junction proteins. Cells were fixed 6-8 days after isolation with ice-cold methanol and subsequently stained for the tight junction proteins Ocln (Sigma-Aldrich, 1:500), ZO-1 (Thermo Fisher Scientific, 1:500), Cldn-5 (Thermo Fisher Scientific, 1:500) and the adherens junction protein VE-Cadherin (Santa Cruz Biotechnology, 1:500). Scale bars represent 50 µm.
Data analysis
Primary mouse brain endothelial cells isolated by this method can be used for a variety of methods that include protein and gene expression analysis, assessment of transendothelial resistance using transwell inserts and transmigration or adhesion assays. In addition, these cells can also be grown on glass coverslips coated with collagen IV for live imaging, e.g., to monitor intracellular calcium dynamics.
Notes
- This protocol was developed to isolate brain endothelial cells from adult mice. We successfully isolated and cultured cells from young mice (6-8 weeks) as well as old mice (up to 1 year) in our laboratory without any modifications to the protocol. Using brains from other mouse strains has not been tested in our laboratory.
- Cells usually reach confluence within 6-8 days (see Figure 4). They can be maintained in culture but are eventually overgrown by pericytes after several weeks.
- After approximately 10 days cells do not adhere as strongly as before and are more likely to detach during staining procedures.
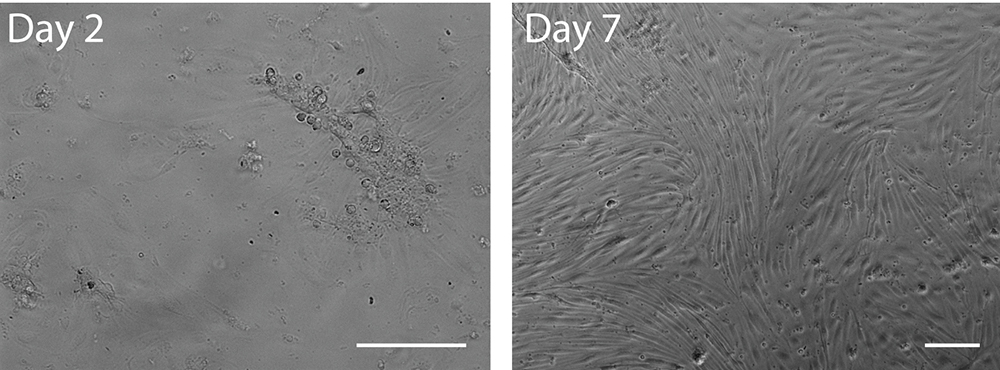
Figure 4. Bright field images of primary murine brain endothelial cells 2 days (left) or 7 days (right) after isolation. Note the attached vessel fragment and its radially outgrowing endothelial cells after 2 days in culture. Scale bars represent 100 µm.
Recipes
- 18% dextran solution (for 2 preparations)
5.4 g dextran dissolved in 30 ml DPBS by heating (microwave)
300 µl penicillin/streptomycin (100x)
300 µl L-glutamine (200 mM)
The solution can be stored at -20 °C and defrosted before usage, but penicillin/streptomycin and L-glutamine should be added after thawing - Working medium (for 2 preparations)
20 ml DMEM-F12
200 µl penicillin/streptomycin (100x)
200 µl L-glutamine (200 mM) - Digestion medium (for 2 preparations)
20 ml DMEM
200 µl penicillin/streptomycin (100x)
200 µl collagenase/dispase–add right before digestion
200 µl TLCK–add right before digestion
80 µl DNase I–add right before digestion - Full medium (max. storage time 3-4 weeks at 4 °C)
40 ml DMEM-F12
10 ml PDS
500 µl antibiotic/antimycotic (100x)
500 µl L-glutamine (200 mM)
150 µl heparin (5,000 U/ml)
500 µl ECGS
Acknowledgments
The protocol described here has been modified based on the method published by Song and Pachter (2003). We would like to thank Beate Lembrich for expert technical assistance. This work was funded by the Deutsche Forschungsgemeinschaft (SCHW416/5-2, 416/9-1).
References
- Ridder, D. A., Wenzel, J., Müller, K., Töllner, K., Tong, X. K., Assmann, J. C., Stroobants, S., Weber, T., Niturad, C., Fischer, L., Lembrich, B., Wolburg, H., Grand'Maison, M., Papadopoulos, P., Korpos, E., Truchetet, F., Rades, D., Sorokin, L. M., Schmidt-Supprian, M., Bedell, B. J., Pasparakis, M., Balschun, D., D’Hooge, R., Löscher, W., Hamel, E. and Schwaninger, M. (2015). Brain endothelial TAK1 and NEMO safeguard the neurovascular unit. J Exp Med 212(10): 1529-1549.
- Song, L. and Pachter, J. S. (2003). Culture of murine brain microvascular endothelial cells that maintain expression and cytoskeletal association of tight junction-associated proteins. In Vitro Cell Dev Biol Anim 39(7): 313-320.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Assmann, J. C., Müller, K., Wenzel, J., Walther, T., Brands, J., Thornton, P., Allan, S. M. and Schwaninger, M. (2017). Isolation and Cultivation of Primary Brain Endothelial Cells from Adult Mice. Bio-protocol 7(10): e2294. DOI: 10.21769/BioProtoc.2294.
- Khan, M. A., Schultz, S., Othman, A., Fleming, T., Lebron-Galan, R., Rades, D., Clemente, D., Nawroth, P. P. and Schwaninger, M. (2016). Hyperglycemia in Stroke Impairs Polarization of Monocytes/Macrophages to a Protective Noninflammatory Cell Type. J Neurosci 36(36): 9313-9325.
Category
Neuroscience > Cellular mechanisms > Cell isolation and culture
Cell Biology > Cell isolation and culture > Cell isolation
Cell Biology > Cell isolation and culture > Cell growth
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










