- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Pathogenicity Assay of Penicillium expansum on Apple Fruits
Published: Vol 7, Iss 9, May 5, 2017 DOI: 10.21769/BioProtoc.2264 Views: 10961
Reviewed by: Zhaohui LiuTohir BozorovAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

In Vitro Hyphal Branching Assay Using Rhizophagus irregularis
Takaya Tominaga and Hironori Kaminaka
Aug 20, 2024 2338 Views

Silencing Arbuscular Mycorrhizal Fungal Gene Using Chitosan Nanoparticle-Mediated dsRNA Delivery System
Chumei Yan [...] Xianan Xie
Jun 5, 2025 2645 Views

A Reliable In Planta Inoculation and Antifungal Screening Protocol for Rhizoctonia solani-Induced Sheath Blight in Rice
Alinaj Yasin [...] Palash Deb Nath
Nov 5, 2025 1582 Views
Abstract
Penicillium expansum, a widespread filamentous fungus, is a major causative agent of fruit decay and leads to huge economic losses during postharvest storage and shipping. Furthermore, it produces mycotoxin on the infected fruits that may cause harmful effects to human health. This pathogenicity assay involves a stab inoculation procedure of P. expansum on apple fruit, an important experimental technique to study fungal pathogenesis. This assay can be applied to analyze the virulence of postharvest pathogen on other fruits such as orange, pear and kiwifruit.
Keywords: Penicillium expansumBackground
Penicillium expansum is a destructive postharvest pathogen that causes decay in many popular fruits, such as apple and pear, during postharvest handling and storage. It causes significant socioeconomic impacts and has implications for international trade. The pathogen can also lead to serious health problems in human since it produces toxic secondary metabolites, including patulin, citrinin, and chaetoglobosins (Andersen et al., 2004). Control of decay caused by P. expansum has become important for ensuring the quality and safety of various fruits.
Conidia of P. expansum typically enter through wounds, which is necessary to provide sites for initiation of the pathogen development (Spotts et al., 1998). Pathogenicity of P. expansum on fruits is usually tested by needle-stab inoculation, which is also used for pathogenicity assays of Botrytis cinerea vs. tomato, Monilinia fructicola vs. peach, Colletotrichum gloeosporioides vs. mango, etc. (Liu et al., 2012; Shi et al., 2012; Zhang et al., 2014). Here, we described a protocol to assess pathogenicity of P. expansum on apple fruits based on stab inoculation method.
Materials and Reagents
- 90 x 15 mm Petri dish (any brand will suffice)
- Plastic film (polyurethane material, any department store)
- 1,000 µl pipette tips (Corning, Axygen®, catalog number: TF-1000-R-S )
- 200 µl pipette tips (Corning, Axygen®, catalog number: TF-200-R-S )
- 10 µl pipette tips (Corning, Axygen®, catalog number: TF-300-R-S )
- Polyester filter cloth cut into 8 x 8 cm squares (any fabric store)
- Penicillium expansum T01: was isolated by our laboratory and whole-genome sequenced (Li et al., 2015)
- Freshly harvested red Fuji apples
- Glycerol (AMRESCO, catalog number: M152 )
- Tween 20 (Sigma-Aldrich, catalog number: T2700 )
- Sodium hypochlorite (Sigma-Aldrich, catalog number: 239305 )
- Sterile distilled water
- Potato
- Dextrose (Macklin, catalog number: D823520 )
- Agar (HUAAOBIO, catalog number: HA0552 )
- 2% sodium hypochlorite solution (see Recipes)
- PDA medium (see Recipes)
Equipment
- Clean bench (Beijing Donglian Har Instrument Manufacture, model: SCB-1520 )
- Sterile nail (approximately 3 mm in diameter, manual polishing)
- Constant temperature incubator (TAICANG, model: THZ-C )
- Hemacytometer (QIUJING, catalog number: XB-K-25 )
- Vortexer (Select BioProducts, model: SBS100-2 )
- 100 µl-1,000 µl pipette (Eppendorf, catalog number: 3120000267 )
- 10 µl-1,00 µl pipette (Eppendorf, catalog number: 3120000240 )
- 0.5µl-1,0 µl pipette (Eppendorf, catalog number: 3120000224 )
- Optical microscope (CHONGQING OPTEC Instrument, model: B203LED )
- 40 x 30 x 10 cm plastic basket (any brand will suffice)
- Hand held sprayer (any brand will suffice)
- 40 x 40 x 30 cm containers (any brand will suffice)
- Hygrothermograph (Fisher Scientific, catalog number: 11-661-20 )
Software
- SPSS version 13.0
Procedure
- Fruit disinfection
- The seasonal apple fruits with uniform size and colour and without physical injuries are used as experimental materials.
- The fruits are surface disinfected for 2 min in a container with 2% sodium hypochlorite solution, rinsed three times with deionized water, and air dried in a clean bench.
- Two wounds (3 x 3 mm) are made face to face with a sterile nail on the equator of each apple prior to inoculation with pathogen (Figure 1A).
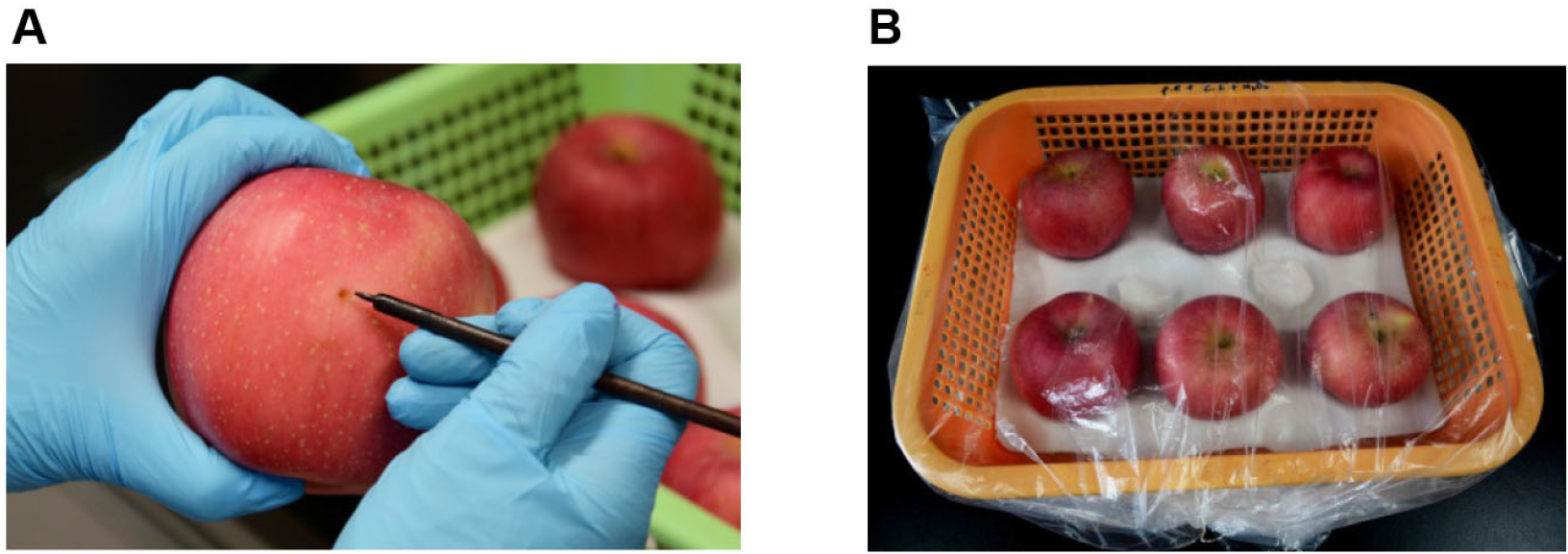
Figure 1. Inoculation of apple fruits with P. expansum. A. Wounding of fruit with sterile nail; B. The storage condition of fruits after inoculation.
- The seasonal apple fruits with uniform size and colour and without physical injuries are used as experimental materials.
- Pathogen inoculum preparation
- Five-microliter spore suspension (5 x 106/ml in 16% glycerol, stored at -80 °C) of P. expansum is inoculated on PDA plate and cultured for 2 weeks at 25 °C in the dark.
- Conidia are harvested with 0.05% Tween 20 and filtered through four layers of sterile polyester filter cloth. Conidia are counted with a hemocytometer using an optical microscope and diluted to a concentration of 1 x 105 conidia/ml with 0.05% Tween 20.
- Five-microliter spore suspension (5 x 106/ml in 16% glycerol, stored at -80 °C) of P. expansum is inoculated on PDA plate and cultured for 2 weeks at 25 °C in the dark.
- Inoculation
- Each wound site of the apple fruits is inoculated with 5 μl spore suspension (the suspension is mixed by vortexer before inoculation) using a pipette. Sterile distilled water with 0.05% Tween 20 is used as the control.
- Six inoculated fruits are put into a plastic basket and sealed with plastic film, about 5 ml sterile water is sprayed on the inside of the plastic film with a hand held sprayer to maintain a high relative humidity (about 95%), and stored at 25 °C in the dark (Figure 1B).
- Each wound site of the apple fruits is inoculated with 5 μl spore suspension (the suspension is mixed by vortexer before inoculation) using a pipette. Sterile distilled water with 0.05% Tween 20 is used as the control.
- Disease scoring
The lesion diameters are measured on a daily basis after three days post-inoculation. Two diameter values of each lesion in two mutually perpendicular directions are recorded. The average of the two values is defined as the diameter of the lesion (Figure 2).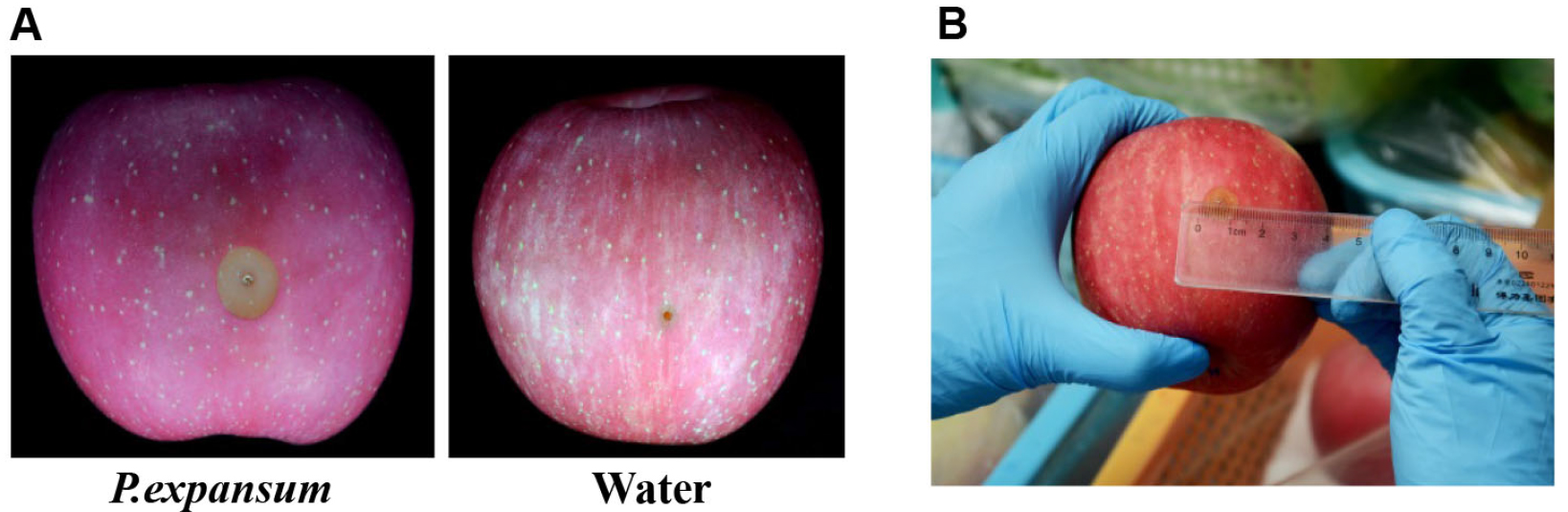
Figure 2. Pathogenicity analysis of infected apple fruits. A. Symptoms of infected apple fruits after inoculation for 4 days at 25 °C; B. Disease scoring of the decayed apple fruits.
Data analysis
Data from three independent experiments, each with 24 fruits (48 wounds), are then analyzed with a statistic software SPSS version 13.0. ANOVA test is performed using Duncan’s multiple range test; P < 0.05.
Representative data
Figure 3 shows representative data for pathogenicity assay of ∆GeneA and ∆GeneB, two gene knock-out mutants of P. expansum, on apple fruits. To compare differences in the virulence of the mutants and WT, the different strains were inoculated into wounds on apple fruits. No significant difference in lesion diameter between ∆GeneA and WT was detected at 4, 5, and 6 d after inoculation (Figures 3A and 3B). Deletion of GeneB significantly reduced the virulence of P. expansum on the fruits. Obvious lesions could be observed 5 days after inoculation, where the lesion diameter of the ∆GeneB was significantly smaller than the lesion size in the wild type (Figuers 3C and 3D). These results indicate that GeneB has a significant impact on virulence of P. expansum.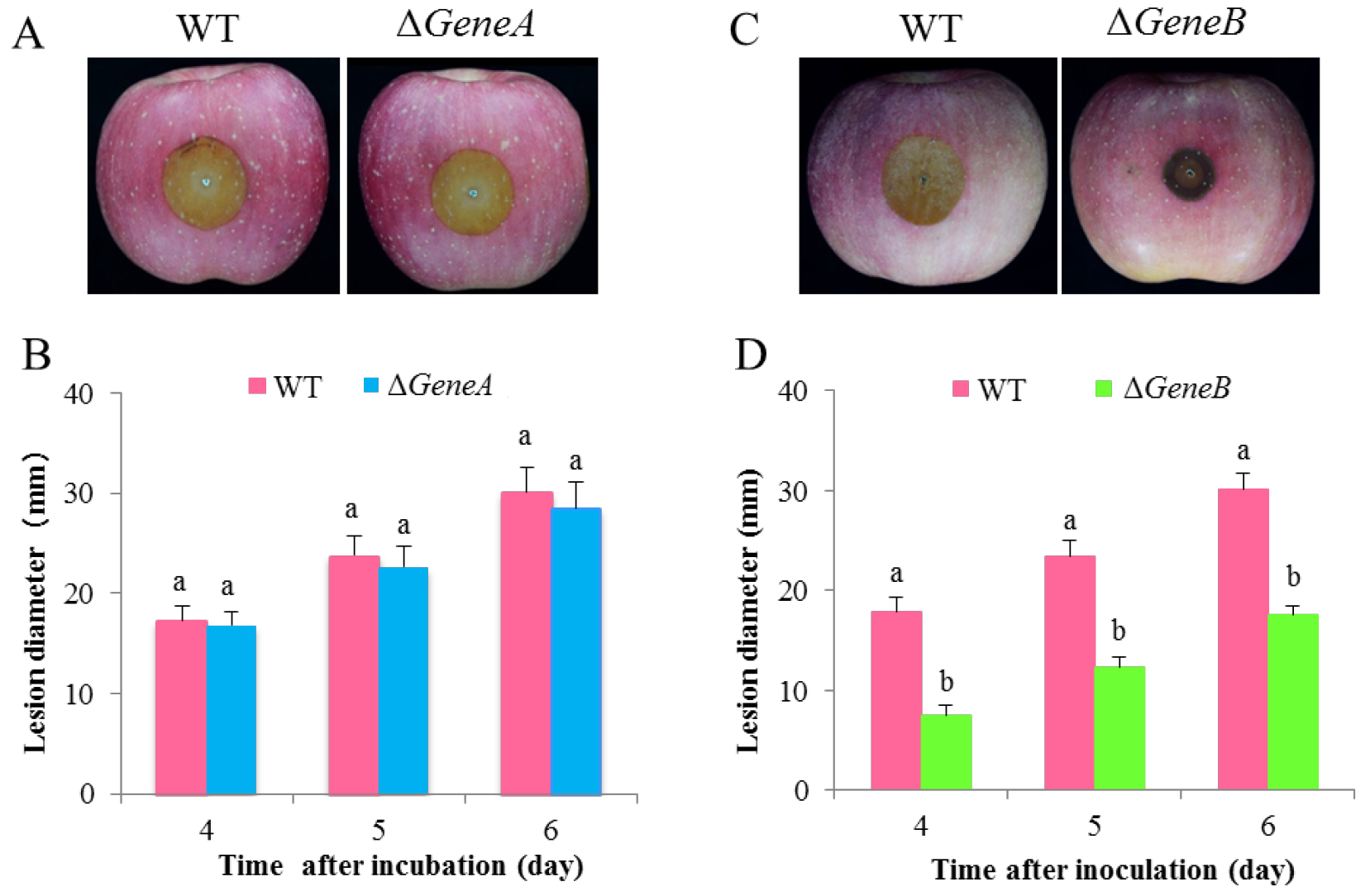
Figure 3. Pathogenicity assay of ∆GeneA and ∆GeneB of P. expansum on apple fruits. A and C. Symptoms of infected apple fruits at 5 d after inoculation; B and D. Statistical analysis of lesion diameters at 4, 5, and 6 d after inoculation at 25 °C.
Notes
- Use 1-2 week old culture to ensure full pathogenicity of P. expansum spores.
- The apple fruits must be fresh and without mechanical wounds.
- The cultivation temperature of infected apple fruits must be controlled at 25 °C or slightly under 25 °C.
Recipes
- 2% sodium hypochlorite solution (20 L)
Add 400 ml of sodium hypochlorite into a 40 x 40 x 30 cm container and then bring volume up to 20 L with distilled water - PDA medium (1 L)
200 g potato
20 g dextrose
15 g agar
Boiling 200 g of sliced potatoes in 1 L distilled water for 30 min, then decanting the broth through cheesecloth and adding 20 g dextrose and 15 g agar powder in the broth. Add distilled water to make up 1 L, and the medium is sterilized by autoclaving at 121 °C for 20 min
Acknowledgments
This protocol was adapted from Li et al. (2015) and Zhang et al. (2014). The work was supported by Chinese Ministry of Science and Technology (grant number 2016YFD0400902).
References
- Andersen, B., Smedsgaard, J. and Frisvad, J. C. (2004). Penicillium expansum: consistent production of patulin, chaetoglobosins, and other secondary metabolites in culture and their natural occurrence in fruit products. J Agric Food Chem 52(8): 2421-2428.
- Li, B., Zong, Y., Du, Z., Chen, Y., Zhang, Z., Qin, G., Zhao, W. and Tian, S. (2015). Genomic characterization reveals insights into patulin biosynthesis and pathogenicity in Penicillium species. Mol Plant Microbe Interact 28(6): 635-647.
- Liu, J., Sui, Y., Wisniewski, M., Droby, S., Tian, S., Norelli, J. and Hershkovitz, V. (2012). Effect of heat treatment on inhibition of Monilinia fructicola and induction of disease resistance in peach fruit. Postharvest Biol Tec 65:61-68.
- Shi, X., Li, B., Qin, G. and Tian, S. (2012). Mechanism of antifungal action of borate against Colletotrichum gloeosporioides related to mitochondrial degradation in spores. Postharvest Biol Tec. 67:138-143.
- Spotts, R., Sanderson, P. G., Lennox, C. L., Sugar, D. and Cervantes, L. A. (1998). Wounding, wound healing and staining of mature pear fruit. Postharvest Biol Tec 13(1): 27-36.
- Zhang, Z., Qin, G., Li, B. and Tian, S. (2014). Knocking out Bcsas1 in Botrytis cinerea impacts growth, development, and secretion of extracellular proteins, which decreases virulence. Mol Plant Microbe Interact 27(6): 590-600.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Chen, Y., Li, B., Zhang, Z. and Tian, S. (2017). Pathogenicity Assay of Penicillium expansum on Apple Fruits. Bio-protocol 7(9): e2264. DOI: 10.21769/BioProtoc.2264.
Category
Plant Science > Plant immunity > Disease bioassay
Microbiology > Microbe-host interactions > Fungus
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link