- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Adoptive Transfer of Lung Antigen Presenting Cells
Published: Vol 7, Iss 6, Mar 20, 2017 DOI: 10.21769/BioProtoc.2182 Views: 11172
Reviewed by: Ivan ZanoniKathrin SutterAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Addressing the Role of Conventional CD8αβ+ T Cells and CD4+ T Cells in Intestinal Immunopathology Using a Bone Marrow–Engrafted Model
Amneh Aoudi [...] Saïdi Soudja
Feb 20, 2024 2968 Views

PBMC-Humanized Mouse Model for Multiple Sclerosis: Studying Immune Changes and CNS Involvement
Anastasia Dagkonaki [...] Lesley Probert
May 20, 2025 3990 Views

Novel Experimental Approach to Investigate Immune Control of Vascular Function: Co-culture of Murine Aortas With T Lymphocytes or Macrophages
Taylor C. Kress [...] Eric J. Belin de Chantemèle
Sep 5, 2025 3550 Views
Abstract
Our protocol describes adoptive transfer of antigen presenting cells (APCs) isolated from the lungs by enzymatic digestion and magnetic enrichment. This protocol can be used to study APC functions and trafficking.
Keywords: Lung antigen presenting cellsBackground
Lung APCs, including macrophages and dendritic cells (DCs), play a critical role in sensing invading pathogens, priming T cell responses and controlling tolerogenic responses. Lung DCs are the most potent professional APCs, including conventional DCs (cDCs) and plasmacytoid DCs (pDCs) during steady-state, and newly recruited monocyte-derived DCs (moDCs) upon inflammation (Kopf et al., 2015). Lung resident macrophages, including alveolar macrophages, interstitial macrophages and bronchial macrophages, are less potent in presenting antigens.
All macrophages and cDCs express high levels of CD11c on their cell surface, while pDCs express intermediate levels of CD11c (Becher et al., 2014). Thus, CD11c magnetic microbeads can be used to isolate mouse lung macrophages and cDCs. We developed a protocol of isolating lung APCs from normal mice and then adoptively transferring them to syngeneic bone marrow transplanted mice. We used this protocol to determine whether normal lung APCs are sufficient to restore T helper cell polarization in response to herpesvirus infection post-transplant (Zhou et al., 2016).
Materials and Reagents
- PrecisionGlide needles 23 G (BD, catalog number: 305111 )
- PrecisionGlide 26 G (BD, catalog number: 305193 )
- 60 x 15 mm Petri dish (Corning, Falcon®, catalog number: 351007 )
- 50 ml flip-top conical centrifuge tubes (Thermo Fisher Scientific, catalog number: 362696 )
- 10 ml Luer slip tip syringe (Fisher Scientific, catalog number: 14-841-55 )
- Razor blade
- Tissue culture plates
- 100 µm nylon screen (Nylon Mesh Lab Pack) (Sefar, catalog number: 7050-1220-000-20 )
- MACS LS column (Miltenyi Biotec, catalog number: 130-042-401 )
- 15 ml conical centrifuge tubes (Corning, Falcon®, catalog number: 352097 )
- 1 ml slip tip syringe (BD, catalog number: 309659 )
- 0.22 µm filter (Millipore Express PLUS 0.22 µm PES) (EMD Millipore, catalog number: SCGPU01RE )
- Pipette tips
- Latex gloves
- Syringe tip caps (BD, catalog number: 305819 )
- Mice
- 70% ethanol
- Phosphate buffered saline (PBS, pH 7.4) (Thermo Fisher Scientific, GibcoTM, catalog number: 10010023 )
- Heat-inactivated fetal bovine serum (Mediatech, catalog number: 35-010-CV )
- Penicillin-streptomycin 100x (Mediatech, catalog number: 30-002-CI )
- L-glutamine 100x, 200 mM (Thermo Fisher Scientific, GibcoTM, 25030-018 )
- Amphotericin B (Thermo Fisher Scientific, GibcoTM, catalog number: 15290018 )
- Dulbecco’s modified Eagle’s medium (DMEM) (Lonza, catalog number: 12-604F )
- Collagenase A (Roche Diagnostics, catalog number: 10103578001 )
- DNase I (Sigma-Aldrich, catalog number: D4263 )
- Ammonium chloride (NH4Cl) (Sigma-Aldrich, catalog number: 254134 )
- Potassium bicarbonate (KHCO3) (Sigma-Aldrich, catalog number: 431583 )
- EDTA, 0.5 M (Lonza, catalog number: 51201 )
- Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A3912 )
- Percoll (Sigma-Aldrich, catalog number: P1644 )
- Mouse CD11c MicroBeads UltraPure (Miltenyi Biotec, catalog number: 130-108-338 )
- Trypan blue solution, 0.4% (Thermo Fisher Scientific, GibcoTM, catalog number: 15250061 )
- Complete media (see Recipes)
- Collagenase digest solution (see Recipes)
- RBC lysis buffer (see Recipes)
- Serum free media (see Recipes)
- MACS buffer (see Recipes)
Equipment
- CO2 tank
- Scissors
- Forceps
- Water bath
- Cell culture incubator
- Rocker
- Refrigerated Swing bucket centrifuge (Beckman Coulter, model: Allegra X-12R )
- Vortex
- Hemocytometer
- Mouse restrainer
- Heat lamp
- Pipette
- Compound microscope
- MACS separator (Miltenyi Biotec)
Procedure
- Preparing single cell suspension from the lungs of donor mice
- Euthanize mice with CO2. Cut the rib cage to expose both the heart and lungs (Figures 1A and 1B).
- To remove blood cells from the lung, perfuse the lungs with 3-5 ml PBS using a 23 gauge needle via the right ventricle of the heart (Figure 1C). Gently inject PBS till the color of lungs turns to white. Note that redness may maintain throughout perfusion in areas inflamed or injured.
- Excise the lung lobes (total 5 per mouse, Figure 1D) and place them in a 60 x 15 mm Petri dish with approximately 1 ml cold PBS and store on ice until all the lungs are harvested from all the mice.
- Remove lungs from PBS and mince with scissors in the lid of the Petri dish until the consistency of applesauce; keep lungs from each mouse separate (Figure 1E).
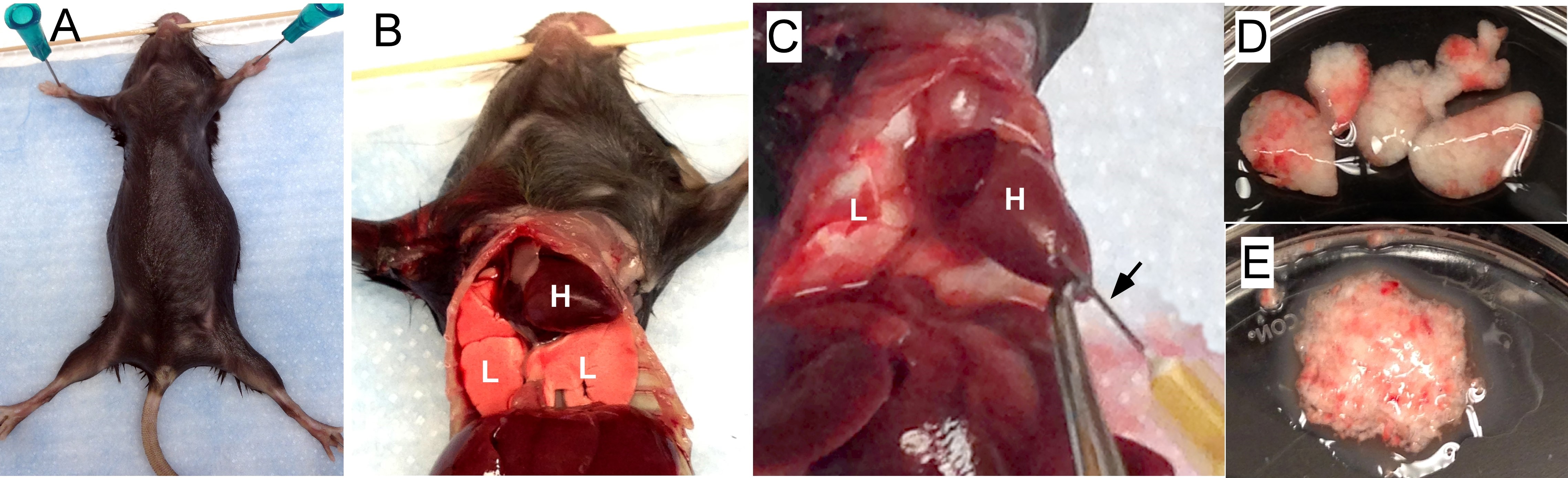
Figure 1. Preparing lung tissue. A. Pin a euthanized mouse on a plastic foam board. B. Cut the rib cage to expose both the heart (H) and the lungs (L). C. Perfuse the lungs by injecting PBS into the right ventricle of the heart. The black arrow points to the needle inserting into the heart. D. Lung lobes (5) are removed and placed in a Petri dish with cold PBS. E. The lungs are minced. - Rinse the scissors and the Petri dish with 15 ml collagenase digest solution (see Recipes below) and place into a 50 ml conical centrifuge tube.
- Close the tubes and incubate the tubes for 5 min in a 37 °C water bath and then for 30 min in a 37 °C incubator on a rocker.
- Using a 10 ml slip tip syringe, draw the sample up and down 20 times to disperse cells.
- Centrifuge for 10 min at 200 x g (i.e., 1,000 rpm) in a swing bucket centrifuge.
- Aspirate the supernatant and discard; break up the pellet by vortexing gently.
- Add 3 ml cold RBC lysis buffer (NH4Cl, see Recipes below) per sample to lyse red blood cells, incubate on ice 2-3 min.
- Add 10 ml cold complete media (see Recipes) to each tube to stop lysis and pellet again at 200 x g for 10 min at 4 °C.
- Aspirate supernatant, break up the pellet, and add 5 ml serum-free media (see Recipes) to resuspend the cells.
- Cut tip off syringe tip cap with razor blade and place it on a 10 ml slip tip syringe (this is to make the syringe tip a little longer for dispersing the volume smaller than 5 ml. Draw the sample up and down 20 times to disperse cells.
- Draw up the solution into the syringe and filter through a sterile 100 µm nylon screen. Rinse the screen with an additional 5 ml of complete media.
- Add 10 ml 40% Percoll in complete media. Mix well by inverting the tubes 5 times and centrifuge for 20 min at 1,800 x g (i.e., 3,000 rpm), turn off brake.
- Aspirate supernatant and discard; break up pellet with 10 ml of complete media. Count cells with trypan blue exclusion on a hemocytometer.
Note: We usually recover 16-17 million cells/naïve mouse.
- Euthanize mice with CO2. Cut the rib cage to expose both the heart and lungs (Figures 1A and 1B).
- Lung APC enrichment
Note: In our hands, the yield of lung APCs is usually about 5% of total cells in the lung single cell suspension. One may roughly calculate a starting total lung cell number in order to obtain enough lung APCs for his/her experiments.- Centrifuge single cell suspension for 10 min at 200 x g and aspirate supernatant and discard.
- Resuspend cell pellet in 400 µl of cold MACS buffer (see Recipes) for less than or equal to 108 total lung cells and add 100 µl of CD11c MicroBeads. Scale up buffer and beads if more than 108 total cells.
- Mix well and incubate for 15 min in the refrigerator (2-8 °C). Note that working on ice may require a longer incubation time.
- Wash cells with 10 ml cold MACS buffer per 108 cells and centrifuge at 200 x g for 10 min at 4 °C.
- Aspirate supernatant and then resuspend cell pellet in 500 µl of MACS buffer.
- Place column in the magnetic field of a MACS LS column. Prepare column by rinsing with 3 ml MACS buffer.
- Place a sterile 100 µm nylon screen on the column. Apply cell suspension onto the column by filtering through the nylon screen. Collect flow-through containing unlabeled cells in a 15 ml conical centrifuge tube.
- Wash column with 3 ml of MACs buffer and collect unlabeled flow-through cells. Wash 3 times.
- Remove column from the separator and place it over a new 15 ml conical centrifuge tube.
- Pipette 5 ml of buffer on the column. Immediately flush out the magnetically labelled cells by firmly pushing the plunger into the column.
- Centrifuge enriched CD11c+ cells at 200 x g for 10 min and discard supernatant.
- Wash cells by gently breaking up the pellet with 10 ml serum free medium. Count cells with trypan blue exclusion on a hemocytometer. Note, usually recover 1-2 million APCs/mouse.
- Centrifuge at 200 x g for 10 min and discard supernatant. Resuspend cells in appropriate volume of serum-free medium to reach a final concentration of 1 x 106 cells per 200 µl.
- Centrifuge single cell suspension for 10 min at 200 x g and aspirate supernatant and discard.
- Adoptive transfer of lung APCs into recipients via intravenous injection
Note: Adoptive transfer can also be achieved by intratracheal administration (for detailed protocol of intratracheal administration, please refer to Ortiz-Muñoz and Looney, 2015, Bio-protocol).- Load cell suspension into a 1 ml syringe with 26 G needle. Make sure to remove air bubbles.
- Place recipient mouse in a plastic mouse restrainer and warm the tail with a heat lamp for about one minute (Figure 2A).
- Immobilize the tail with gentle traction (Figure 2A).
- Spray the tail with 70% ethanol to visualize the lateral tail vein and rotate the tail ¼ turn for easier injection.
- At the distal portion of the tail, insert needle parallel to the vein 2 to 5 mm into to the lumen (Figure 2B).
- Inject 1 x 106 cells in 200 µl per mouse. Inject the cell suspension slowly. The cell suspension should flow easily if needle is properly located.
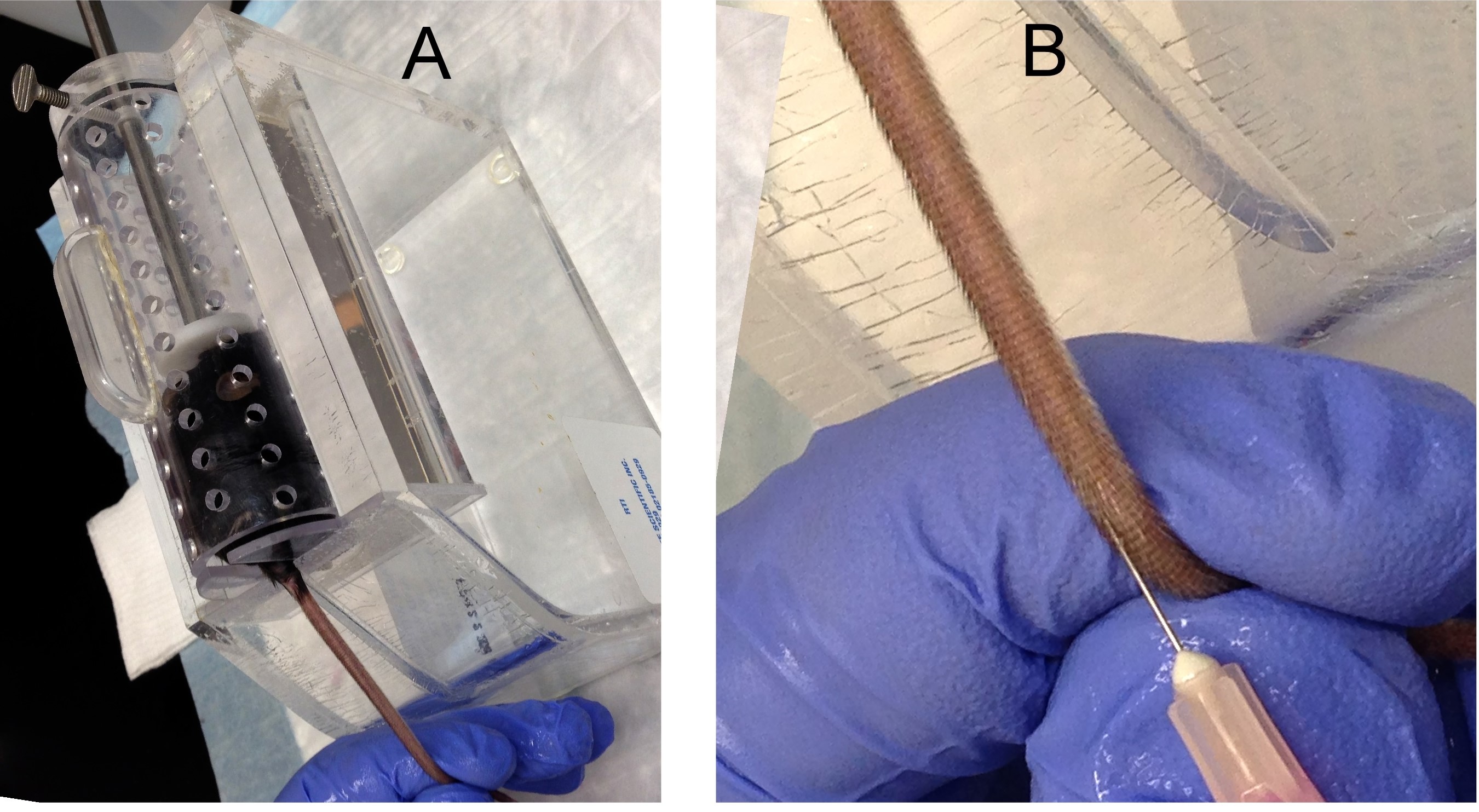
Figure 2. Tail vein injection. A. A recipient mouse is placed in a restrainer. B. Insert a needle into a mouse tail vein to inject cells. - Withdraw the needle and apply pressure to prevent bleeding.
- Place the recipient mouse back into its cage.
- Mice may then receive designated treatments and can be analyzed at appropriate time points.
- Load cell suspension into a 1 ml syringe with 26 G needle. Make sure to remove air bubbles.
Data analysis
Single cell suspension was prepared from the lungs of C57BL/6 mice three days post infection of murine gamma herpesvirus 68 (MHV-68) intranasal at a dose of 5 x 104 pfu/mouse (Zhou et al., 2016). CD11c+ cells were then enriched by using anti-mouse CD11c microbeads. To analyze the composition of these microbead-enriched cells by flow cytometry, appropriate fluorescence-conjugated antibodies were used to stain cell surface markers. Percentages on each plot represent the percentages of each parental gate. Similar results were obtained in two additional independent experiments. The majority enriched CD11c+ APCs are alveolar macrophages (AMs) (CD45+ CD11c+ MHC IIint CD64hi), and DCs. The DC population can be further separated into CD103 cDCs (CD45+ CD11c+ MHC IIhi CD64- CD103hi CD11bint), CD11b+ cDCs (CD45+ CD11c+ MHC IIhi CD64- CD103lo CD11bhi) and MoDCs (CD45+ CD11c+ MHC IIhi CD64+ CD103lo CD11bhi) (Misharin et al., 2013; Wang et al., 2006) (Figure 3) .
Lung APCs were prepared from congenic CD45.1 mice three days post infection of MHV-68, and adoptively transferred into wild-type C57BL/6 mice (CD45.2+, but CD45.1-). The recipients were infected with MHV-68 24 h after cell transfer, and euthanized for flow cytometry analysis 48 h after cell transfer. Representative flow cytometry plots show donor CD45.1+ APCs in the lungs, lung draining lymph nodes (dLN) and spleens of CD45.1- recipients (Figure 4).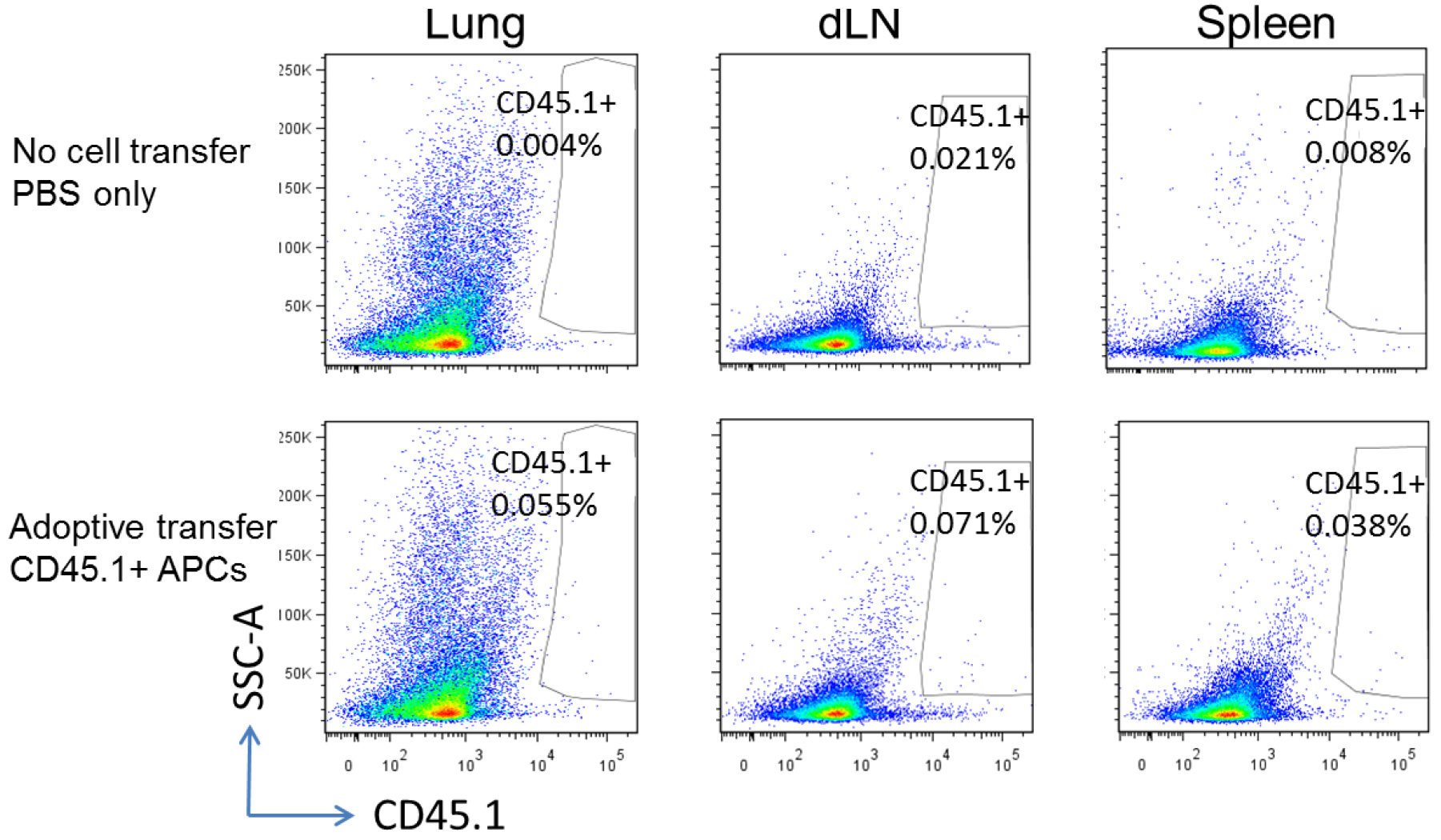
Figure 3. Representative flow cytometry plots of APCs isolated from mouse lungs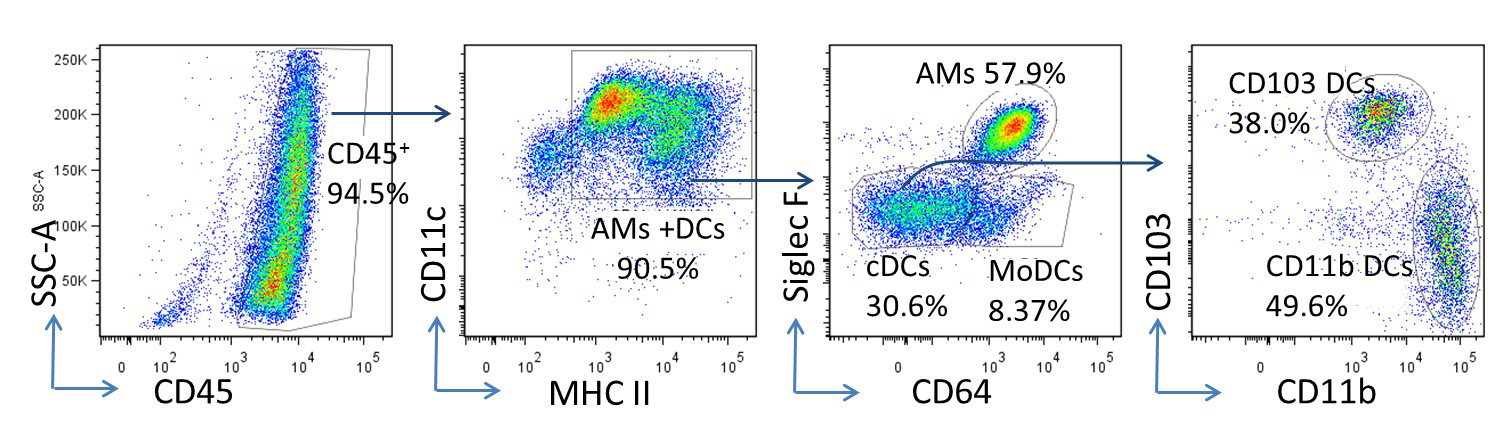
Figure 4. Tracking injected cells. Upper panel, wild-type C57BL/6 mice without cell transfer; lower panel, wild-type C57BL/6 recipients adoptively transferred with CD45.1+ lung APCs.
Notes
If CD11c+ subpopulations are under investigation, the magnetically enriched cells can be further stained with appropriate fluorescence-conjugated antibodies and sorted to specific APC populations using fluorescence-activated cell sorting (FACS) (Misharin et al., 2013).
Recipes
- Complete media
Mix the following solutions and filter through 0.22 µm filter:
50 ml heat-inactivated fetal bovine serum
5 ml 100x penicillin-streptomycin (10,000 IU penicillin and 10,000 µg/ml streptomycin)
5 ml 100x L-glutamine (200 mM)
0.5 ml amphotericin B (250 µg/ml)
Add the filtered mixture into a 500 ml bottle of DMEM media - Collagenase digest solution (per mouse, prepare immediately before use)
15 mg collagenase A
0.25 ml DNase I (250 Kunitz unit)
15 ml complete media - RBC lysis buffer
Dissolve the following in 800 ml distilled H2O
8.3 g NH4Cl
1.0 g KHCO3
1.8 ml of 5% EDTA
Add distilled H2O up to a total volume of 1,000 ml
Filter sterilize through a 0.22 µm filter, and keep in refrigerator - Serum free media
500 ml DMEM media
5 ml 10% BSA
5 ml 100x penicillin-streptomycin (10,000 IU penicillin and 10,000 µg/ml streptomycin)
5 ml 100x L-glutamine (200 mM)
0.5 ml amphotericin B (250 µg/ml)
Store in refrigerator (4-8 °C) - MACS buffer
For 150 ml solution:
7.5 ml of 10% bovine serum albumin (BSA)
0.6 ml of 0.5 M EDTA
Store in refrigerator (4-8 °C)
Acknowledgments
This protocol was adapted from our publication (Zhou et al., 2016). This work was supported by NIH grants AI117229, HL115618, T32HL07749 and 2UL1TR000433.
References
- Becher, B., Schlitzer, A., Chen, J., Mair, F., Sumatoh, H. R., Teng, K. W., Low, D., Ruedl, C., Riccardi-Castagnoli, P., Poidinger, M., Greter, M., Ginhoux, F. and Newell, E. W. (2014). High-dimensional analysis of the murine myeloid cell system. Nat Immunol 15(12): 1181-1189.
- Kopf, M., Schneider, C. and Nobs, S. P. (2015). The development and function of lung-resident macrophages and dendritic cells. Nat Immunol 16(1): 36-44.
- Misharin, A. V., Morales-Nebreda, L., Mutlu, G. M., Budinger, G. R. and Perlman, H. (2013). Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. Am J Respir Cell Mol Biol 49(4): 503-510.
- Ortiz-Muñoz, G. and Looney, M. R. (2015). Non-invasive intratracheal instillation in mice. Bio-protocol 5(12): e1504.
- Wang, H., Peters, N., Laza-Stanca, V., Nawroly, N., Johnston, S. L. and Schwarze, J. (2006). Local CD11c+ MHC class II- precursors generate lung dendritic cells during respiratory viral infection, but are depleted in the process. J Immunol 177(4): 2536-2542.
- Zhou, X., Loomis-King, H., Gurczynski, S. J., Wilke, C. A., Konopka, K. E., Ptaschinski, C., Coomes, S. M., Iwakura, Y., van Dyk, L. F., Lukacs, N. W. and Moore, B. B. (2016). Bone marrow transplantation alters lung antigen-presenting cells to promote TH17 response and the development of pneumonitis and fibrosis following gammaherpesvirus infection. Mucosal Immunol 9(3): 610-620.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Zhou, X. and Moore, B. B. (2017). Adoptive Transfer of Lung Antigen Presenting Cells. Bio-protocol 7(6): e2182. DOI: 10.21769/BioProtoc.2182.
Category
Immunology > Immune cell function > Macrophage
Cell Biology > Cell Transplantation > Immune cell adoptive transfer
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









