- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Reprogram Murine Epiblast Stem Cells by Epigenetic Inhibitors
Published: Vol 7, Iss 5, Mar 5, 2017 DOI: 10.21769/BioProtoc.2168 Views: 7399
Reviewed by: Nicoletta CordaniZhen ShiAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Differentiation, Maintenance, and Contraction Profiling of Human Induced Pluripotent Stem Cell–Derived Cardiomyocytes
Matthijs Snelders [...] Jeroen Essers
Mar 5, 2025 3937 Views

Isolation and Culture of Ferret Airway Stem Cells
Ziying Yan [...] Feng Yuan
Jul 20, 2025 2413 Views

Optimization of Adipogenic Differentiation Protocol for Murine and Human Cell Culture Models
Junwan Fan [...] Wenyan He
Jan 20, 2026 230 Views
Abstract
Pluripotent stem cells in the naïve state are highly useful in regenerative medicine and tissue engineering. A robust reprogramming of the primed murine Epiblast Stem Cells (EpiSCs) to naïve pluripotency is feasible via chemical-only approach. This protocol described a method to reprogram murine EpiSCs by MM-401 treatment, which blocks histone H3K4 methylation by MLL1/KMT2A.
Keywords: Naïve pluripotencyBackground
Previous protocols on EpiSC reprogramming depended on the genetic manipulations of transcriptional factors or chemical inhibition of the signaling pathways, albeit with varying efficiency and duration. Based on a recent mechanistic study that links the MLL1 complex to the naïve state, this protocol provides a straightforward and robust method to restore naïve pluripotency from EpiSCs via targeting MLL1 mediated H3K4 methylation and subsequent transcription regulation. The reprogramming efficiency is significantly higher than previous published method, achieving 50% conversion rate in two weeks.
Materials and Reagents
- Tissue culture plate, 24 well (Corning, Falcon®, catalog number: 353047 )
- 15 ml conical tube (Corning, Falcon®, catalog number: 352196 )
- MEF feeder cells (University of Michigan, Transgenic Animal Model Core, https://www.med.umich.edu/tamc/list.html#reagents)
- 0.2% gelatin solution (Sigma-Aldrich, catalog number: G1890 )
- PBS
- Collagenase type IV (Thermo Fisher Scientific, GibcoTM, catalog number: 17104-019 )
- 0.05% trypsin (Thermo Fisher Scientific, GibcoTM, catalog number: 25300-054 )
- The VectorTM alkaline phosphatase (AP) staining kit (Vector Laboratories, catalog number: SK-5100 )
- Dulbecco’s modified Eagle medium (DMEM) (Thermo Fisher Scientific, GibcoTM, catalog number: 11995-065 )
- Fetal bovine serum (FBS) (Atlas Biologicals, catalog number: F-0500-D )
- L-glutamine (200 mM) (Thermo Fisher Scientific, GibcoTM, catalog number: 25030-081 )
- 100x non-essential amino acids (Thermo Fisher Scientific, GibcoTM, catalog number: 11140-050 )
- 100x sodium pyruvate (Thermo Fisher Scientific, GibcoTM, catalog number: 11360-070 )
- 2-mercaptoethanol (Sigma-Aldrich, catalog number: M7522 )
Note: This product has been discontinued. - KnockOutTM D-MEM (Thermo Fisher Scientific, GibcoTM, catalog number: 10829018 )
- KnockOutTM serum replacement (KSR) (Thermo Fisher Scientific, GibcoTM, catalog number: 10828028 )
- Glutamax (Thermo Fisher Scientific, GibcoTM, catalog number: 35050061 )
- Fibroblast growth factor basic (FGF2), human recombinant (R&D Systems, catalog number: 233-FB )
- ESGRO® leukemia inhibitory factor (LIF) (EMD Millipore, catalog number: ESG1107 )
- MM-401
- DMSO
- Mouse embryonic fibroblasts (MEFs) culture medium (see Recipes)
- EpiSCs culture medium (see Recipes)
- Embryonic stem cells (ESCs) culture medium (see Recipes)
- 100 mM MM-401 (see Recipes)
- MM-401 reversion medium (see Recipes)
- MM-401 maintenance medium (see Recipes)
Equipment
- 1 ml pipette
- Tissue culture incubator (IsotempTM Microbiological Incubator) (Fisher Scientific, model: Fisher ScientificTM IsotempcatalogTM Microbiological Incubator, catalog number: S28670 ), humidified, 37 °C and 5% CO2 in air
- Stereomicroscope (Olympus, model: DP73 )
- Centrifuge (Eppendorf, model: 5810 R ; Swing-bucket rotor: Eppendorf, model: A-4-81 )
Procedure
A schematic summary of the reprogramming procedure described in this protocol can be found in Figure 1.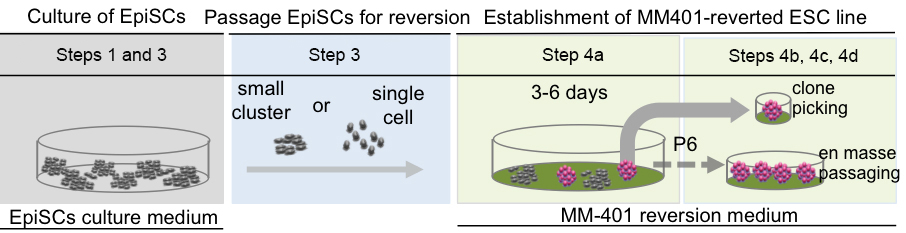
Figure 1. The schematic view of general procedures for the reversion of EpiSCs by MM-401 treatment
- Preparation of MEF feeder cells: Irradiated MEF feeder cells are seeded at 1 x 105 cell/well in MEF medium in a 24-well plate (pre-coated with 0.2% gelatin solution).
- EpiSC culture: EpiSCs are routinely cultured following previously described protocol (Chenoweth and Tesar, 2010). For chemical treatment experiments, EpiSCs are cultured in the 24-well plate and enzymatically passaged every third day (see Figure 2).
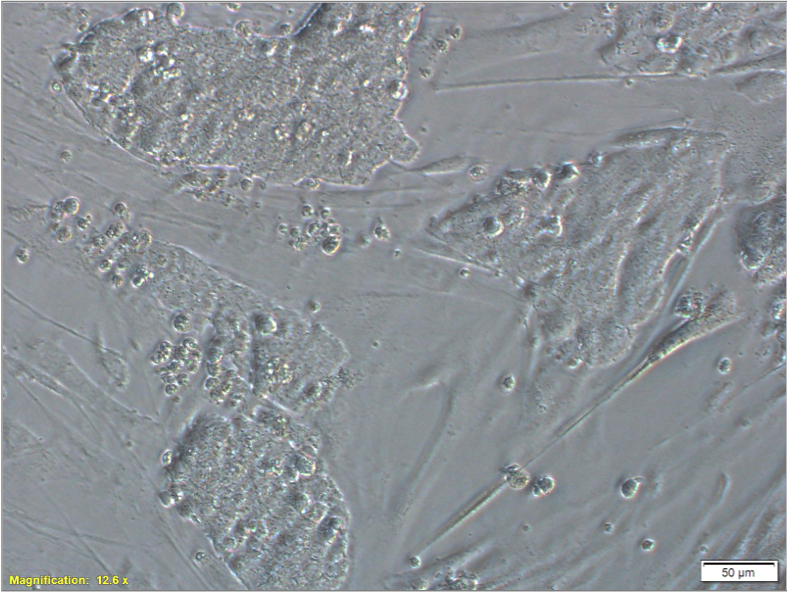 Figure 2. Representative phase image of EpiSCs at the third day of culture before splitting. Scale bar = 50 μm.To passage,
Figure 2. Representative phase image of EpiSCs at the third day of culture before splitting. Scale bar = 50 μm.To passage,- Remove EpiSC culture medium from each well and wash briefly with PBS. Discard PBS and add 150 μl of collagenase type IV or 0.05% trypsin to each well, and incubate at room temperature for up to 4 min.
- Add 1 ml of EpiSC culture medium (without FGF2) to each well and gently pipet with a 1-ml pipette. Combine suspensions from 1 to 2 wells into a 15-ml conical tube. Add EpiSC culture medium (without FGF2) to a final volume of 5 ml to each tube for centrifugation.
- Separate EpiSCs from MEFs (Optional):
- For EpiSCs passaging in small cluster, separate clusters by centrifugation at 8 x g for 15 sec (25 °C). The EpiSC clusters will loosely pellet while the individual MEFs remain in the supernatant.
- For EpiSCs passaging in single cell, separate EpiSCs by centrifugation at 8 x g for 3 min (25 °C). A mixture of the MEFs and EpiSCs will loosely pallet while the individual single EpiSC will remain in the supernatant.
- Examine the separated EpiSCs after centrifugation under the microscope. Single EpiSCs and remaining MEF feeder cells can be distinguished by size (see Figure 3).
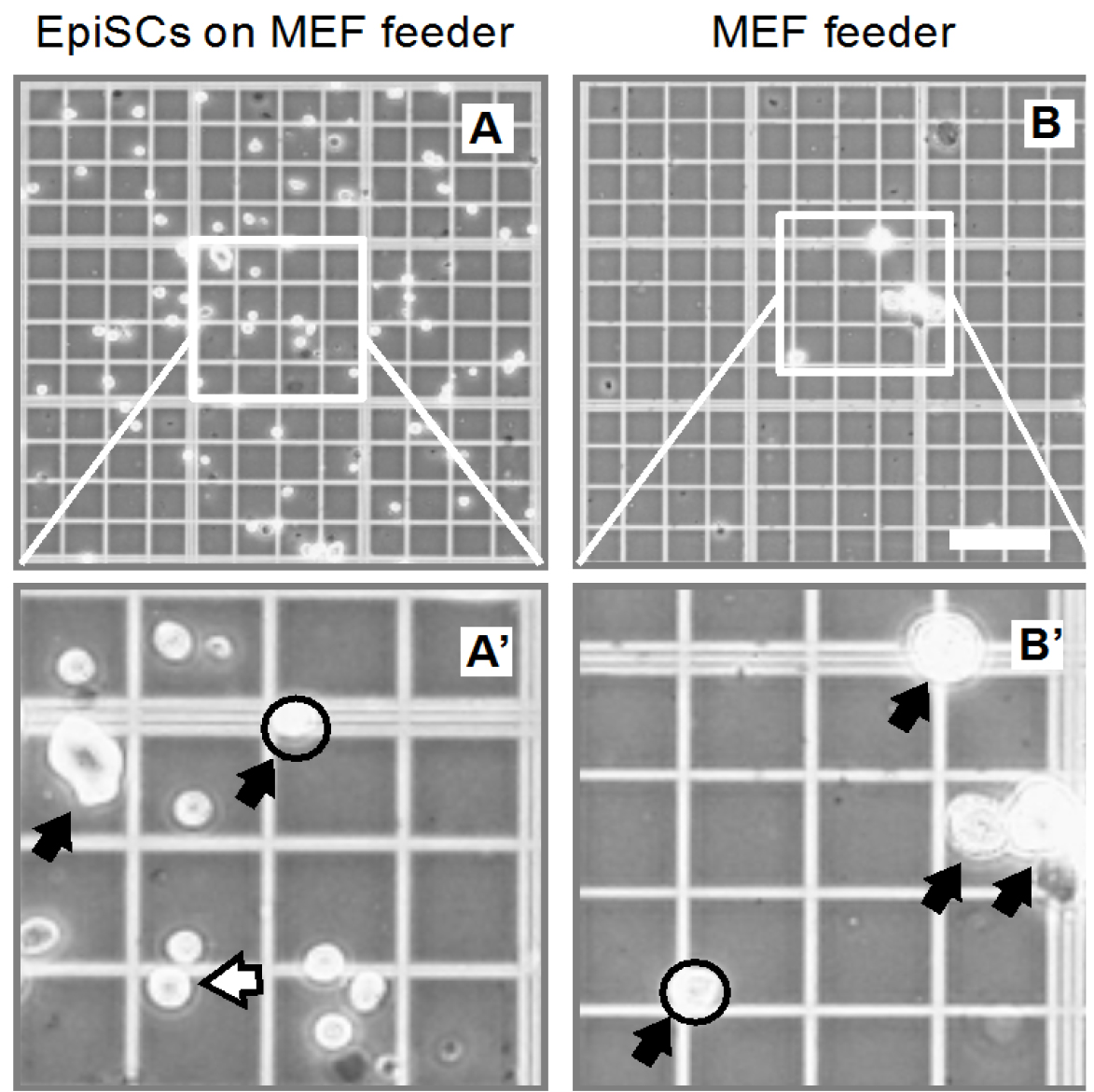
Figure 3. Representative image shows the EpiSCs and MEF feeders (collected from 2 well of 24-well plate) before and after centrifugation under the microscope. A and A’. EpiSCs and MEF feeder before separation; B and B’. MEF feeder cells. Black arrow, MEF feeder; Open arrow, EpiSC cells; Open circle, MEF feeder cells, which are still 2-3-fold larger than single EpiSC cell. Scale bar = 100 μm.
- Remove EpiSC culture medium from each well and wash briefly with PBS. Discard PBS and add 150 μl of collagenase type IV or 0.05% trypsin to each well, and incubate at room temperature for up to 4 min.
- Plating of EpiSCs for reversion: EpiSCs were passaged in single cell or small clumps on MEF feeder cells (as described in step 1) in MM-401 reversion medium.
- Centrifuge the EpiSCs (from step 2b or 2c), aspirate and discard the supernatant. Resuspend EpiSC pellet in MM-401 reversion medium. For the 24-well plate, add 400 μl MM-401 reversion medium to each well.
- For EpiSCs passaging in small cluster, replate cells to a new well with 1:4-1:5 dilutions. For EpiSCs passaging in single cells, plate the cells at 20,000/cm2 (approximately 4 x 104 cell/well for the 24-well plate, see Figure 4).
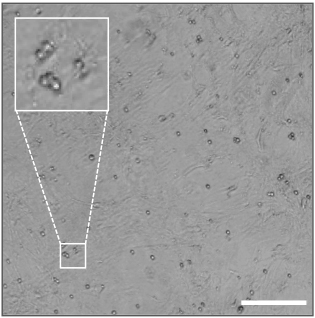
Figure 4. Representative image of single EpiSCs plated at the density of 20,000 cell/cm2. Scale bar = 200 μm.
- Centrifuge the EpiSCs (from step 2b or 2c), aspirate and discard the supernatant. Resuspend EpiSC pellet in MM-401 reversion medium. For the 24-well plate, add 400 μl MM-401 reversion medium to each well.
- Establish MM-401-reverted ESC line
- Morphologically distinct reverted naïve colonies become evident over the next 3-6 days. AKP is used to stain the colonies to evaluate reversion efficiency. Strong AKP staining distinguishes the reverted naïve colonies from EpiSCs that have weak AKP staining (see Figure 5). AKP activity is detected using VectorTM alkaline phosphatase (AP) staining kit according to the manufacturer’s instructions.
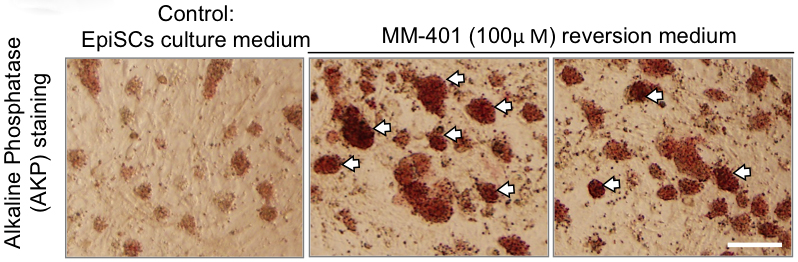
Figure 5. AKP staining of EpiSCs and reverted naïve colonies after MM-401 treatment as indicated on top. Left: Flat EpiSC clones with weak AKP staining before MM401 induction; Right: Two representative images of EpiSCs taken at 72 h of 100 μM MM-401 treatment. The reverted cells (indicated by arrows) are morphologically distinct from EpiSCs. The colonies are dome-shaped with intense AKP staining. Scale bar = 100 μm - The reversion culture is passaged by a brief exposure (4-5 min) to 0.05% trypsin/EDTA with gentle pipetting into single cells. Cells can be re-seeded at 20,000-50,000/cm2. MM-401 is replenished to reach 50-100 μM final concentrations in each passage.
- Reverted ESC (rESC) lines can be established by clone picking or en masse passaging. The established rESC line can be maintained, passaged, cryopreserved and thawed by the standard protocol for conventional mESCs.
- rESC line can be expanded in MM-401 maintenance medium or ESC culture medium without MM-401 after passage 6.
Notes
- For reversion, MM-401 (50-100 μM final concentration) can be added to culture medium immediately or 2-3 days after EpiSCs forming clones.
- Mediums containing MM-401 are recommended to be pre-warmed at room temperature (RT) for 15 min.
- For MM-401 request: please contact yalid@med.umich.edu.
Recipes
- Mouse embryonic fibroblasts (MEFs) medium
DMEM supplemented with:
10% FBS
2 mM 1x L-glutamine
1x nonessential amino acids
1x sodium pyruvate
0.1 mM 2-mercaptoethanol - EpiSC culture medium
KnockOutTM D-MEM supplemented with:
20% KSR
2 mM Glutamax
1x nonessential amino acids
1x sodium pyruvate
0.1 mM 2-mercaptoethanol
10 ng/ml FGF2
Note: The medium can be stored at 4 °C for up to 2 weeks. - Embryonic stem cells (ESCs) culture medium
KnockOutTM D-MEM supplemented with:
20% FBS
2 mM Glutamax
1x nonessential amino acids
1x sodium pyruvate
0.1 mM 2-mercaptoethanol
103 U/ml LIF
Note: The medium can be stored at 4 °C for up to 2 weeks. - 100 mM MM-401
MM-401 (Molecular Mass: 700.75) is dissolved in DMSO at the final concentration of 100 mM
Note: It is stored at -20 °C or -80 °C. - MM-401 reversion medium
ESC culture medium supplemented with 100 μM MM-401
Note: The medium can be stored at 4 °C for up to 2 weeks. - MM-401 maintenance medium
ESC culture medium supplemented with 20 μM MM-401
Note: The medium can be stored at 4 °C for up to 2 weeks.
Acknowledgments
This protocol was originally published as part of Zhang et al. (2016). The authors wish to thank all present and past members developing/functional testing MM-401 in the Dr. Yali Dou, Dr. Sundeep Kalantry and Dr. Shaomeng Wang laboratories. Special thanks are obligated to Dr. Saunders and Ms. Hughes at the University of Michigan for technical support, and Dr. Brady and Dr. Tesar for sharing materials.
This work is supported by National Institute of General Medical Sciences (NIGMS) (GM082856), Leukemia and Lymphoma Society and the National Cancer Institute (CA117307-04) to Dr. Yali Dou.
References
- Chenoweth, J. G. and Tesar, P. J. (2010). Isolation and maintenance of mouse epiblast stem cells. Methods Mol Biol 636: 25-44.
- Zhang, H., Gayen, S., Xiong, J., Zhou, B., Shanmugam, A. K., Sun, Y., Karatas, H., Liu, L., Rao, R. C., Wang, S., Nesvizhskii, A. I., Kalantry, S. and Dou, Y. (2016). MLL1 inhibition reprograms epiblast stem cells to naive pluripotency. Cell Stem Cell 18(4): 481-494.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Zhang, H. and Dou, Y. (2017). Reprogram Murine Epiblast Stem Cells by Epigenetic Inhibitors. Bio-protocol 7(5): e2168. DOI: 10.21769/BioProtoc.2168.
Category
Stem Cell > Pluripotent stem cell > Cell induction
Stem Cell > Embryonic stem cell > Maintenance and differentiation
Cell Biology > Cell isolation and culture > Cell differentiation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








