- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Laser Scanning Confocal Microcopy for Arabidopsis Epidermal, Mesophyll, and Vascular Parenchyma Cells
(*contributed equally to this work) Published: Vol 7, Iss 5, Mar 5, 2017 DOI: 10.21769/BioProtoc.2150 Views: 14586
Reviewed by: Arsalan DaudiAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Live Leaf-Section Imaging for Visualizing Intracellular Chloroplast Movement and Analyzing Cell–Cell Interactions
Yuta Kato [...] Mitsutaka Taniguchi
Aug 5, 2025 2329 Views

Live-Cell Monitoring of Piecemeal Chloroplast Autophagy
Masanori Izumi [...] Shinya Hagihara
Nov 5, 2025 1695 Views

Chloroplast Movement Imaging Under Different Light Regimes With a Hyperspectral Camera
Paweł Hermanowicz [...] Justyna Łabuz
Dec 20, 2025 759 Views
Abstract
Investigation of protein targeting to plastids in plants by confocal laser scanning microscopy (CLSM) can be complicated by numerous sources of artifact, ranging from misinterpretations from in vivo protein over-expression, false fluorescence in cells under stress, and organellar mis-identification. Our studies have focused on the plant-specific gene MSH1, which encodes a dual targeting protein that is regulated in its expression and resides within the nucleoid of a specialized plastid type (Virdi et al., 2016). Therefore, our methods have been optimized to study protein dual targeting to mitochondria and plastids, spatial and temporal regulation of protein expression, and sub-organellar localization, producing a protocol and set of experimental standards that others may find useful for such studies.
Keywords: ConfocalBackground
Protein targeting behavior in plants is influenced by amino-terminal presequences as well as internal sequence features that can influence suborganellar localization behaviors (Baginsky and Gruissem, 2004). Combined with promoter-driven spatial and temporal regulation in expression, a protein’s activity can be extremely precise and specialized by virtue of timing and location. In the case of MSH1, this nuclear-encoded, plant-specific protein is dual targeted to mitochondria and plastids (Xu et al., 2011). Promoter features direct its expression to reproductive, epidermal and vascular parenchyma cells (Virdi et al., 2016). Internal protein features direct its localization to the mitochondrial and plastid nucleoid, as well as to the plastid thylakoid membrane. Discovery of these unusual protein features was greatly facilitated by laser scanning confocal microscopy using methodologies described here. Much of this detail would have been overlooked using more traditional organellar subfractionation methodologies.
Materials and Reagents
- 5 ml tube
- Needleless syringe
- Double edge razor blades (Electron Microscopy Sciences, PersonnaTM, catalog number: 72000 )
- Glass cover slips, No. 1.5 (VWR, catalog number: 16004-302 )
- Dissecting needle or probe pin
- Petri dish (VWR, catalog number: 25384-302 )
- Centrifuge tube (VWR, catalog number: 89039-668 )
- 0.45 μm filter (VWR, catalog number: 28145-481 )
- Disposable transfer pipette (Fisher Scientific, catalog number: 13-7117M )
- Glass slides (VWR, catalog number: 48300-048 )
- Arabidopsis leaf/flower/stem tissue (Ecotype Col-0, 6 weeks old plants)
- Lurie broth
- Tween 20 (Sigma-Aldrich, catalog number: P1379 )
- Incubation solution
- Potassium phosphate dibasic (K2HPO4) (Sigma-Aldrich, catalog number: P3786 )
- Potassium phosphate monobasic (KH2PO4) (Sigma-Aldrich, catalog number: P5655 )
- Ammonium sulfate, (NH4)2SO4 (Sigma-Aldrich, catalog number: A4418 )
- Sodium citrate dihydrate (Sigma-Aldrich, catalog number: C8532 )
- Magnesium sulfate (MgSO4) (1 M stock solution) (Sigma-Aldrich, catalog number: M2643 )
- Glucose (Sigma-Aldrich, catalog number: G8270 )
- Glycerol (Sigma-Aldrich, catalog number: G5516 )
- MES (Sigma-Aldrich, catalog number: M2933 )
- KOH or NaOH
- Acetosyringone (3’,5’-dimethoxy-4’-hydroxyacetophenone) (Sigma-Aldrich, catalog number: D134406 )
- MS medium basal salts (Sigma-Aldrich, catalog number: M5519 )
- Cellulase ‘onozuka’ R-10 (Yakult Honsha, Tokyo, Japan)
- Macerozyme R-10 (Yakult Honsha, Tokyo, Japan)
- Mannitol (Sigma-Aldrich, catalog number: M1902 )
- Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P9541 )
- Calcium chloride dihydrate (CaCl2·2H2O) (Sigma-Aldrich, catalog number: C7902 )
- BSA (optional) (Sigma-Aldrich, catalog number: A7906 )
- Induction medium (see Recipes)
- Infiltration medium (see Recipes)
- Cellulase/macerozyme solution (see Recipes)
- Washing and incubation solution (WI) (see Recipes)
Equipment
- 387/478/555 nm triple-bandpass filter excitation with a broad emission filter ~400-700 nm
- LED illumination, 488 nm, 543 nm (Lumencor AURA light engine)
- Incubator shaker
- Platform shaker
- Forceps (VWR, catalog number: 82027-440 ) or (Cole-Parmer Instrument, catalog number: EW-07287-09 )
- 90i upright compound microscope (Nikon Instruments)
- 10x Plan Apo 0.45NA (Nikon Instruments, model: OFN25 )
- 20x Plan Apo 0.75NA (Nikon Instruments, model: lambda )
- 60x Plan Apo VC water immersion lens 1.2NA (Nikon Instruments, model: MRD07602 )
- Confocal laser scanning microscope (CLSM) (Nikon Instruments, model: A1+ )
- Autoclave
- Water bath
- Vacuum desiccator
Software
- NIS-Elements software
Procedure
- Agrobacterium mediated transient transformation of tobacco leaves
- Culture Agrobacterium strain C58C1 carrying the target gene of interest in a 5 ml culture medium of Lurie broth overnight at 30 °C in an incubation shaker and spin down at 1,000 x g.
- Transfer the bacterial cells into 5 ml of induction medium (see Recipes) and grow at 30 °C for 16 h with shaking.
- Spin the cells down and re-suspended in 1 ml infiltration medium (see Recipes).
- Measure the bacterial concentration to an optical density of 0.8 at 600 nm in a fresh 5 ml tube of infiltration medium. Infiltrate young leaves of about 4 weeks old tobacco plants, Nicotiana tabacum cv. xanthi using a needleless syringe. Visualize plant expression after 48 h.
- Culture Agrobacterium strain C58C1 carrying the target gene of interest in a 5 ml culture medium of Lurie broth overnight at 30 °C in an incubation shaker and spin down at 1,000 x g.
- Preparation of Arabidopsis plant samples
- GFP or YFP when viewed through the oculars with a broad emission filter coupled with a 488 nm LED will not only allow visualization of GFP or YFP (with less excitation efficiency) but also the plastids. This full spectrum visualization allows the user to differentiate damaged areas where plastids shift from red emission to orange. Furthermore in variegated tissues, ocular observation may be the only method of differentiating low GFP or YFP and damaged/stressed areas. Ocular observations have the benefit of much deeper optical sections, providing context for localization. In this case, 10x and 20x visualization provided clues to pursue vascular localization.
- RFP excited with 543 nm light visualized with a broad emission filter will appear a deep orange color in a ruby red background from the plastids. With low RFP expression, it’s difficult to differentiate the two, and the CSLM should be used to separate the two.
- The benefit of using LED over more common mercury vapor lamps is the reduction in photobleaching or degradation of the plastid pigments, which will shift emission causing erroneous analyses.
- Leaf/flower
The surface tension of the water used for wet mounting under a larger cover slip will hold samples steady during imaging. The addition of a trace of Tween 20 (5-10 µl in 250 ml water) will assist in the wetting of flowers, which are far more hydrophobic than leaves. - Stems
A double-edged razor blade snapped in half longitudinally before unwrapping is used to make cross sections of stems. Snapping double-edged razor blades is hazardous (Figure 1). The stem sections should be taken in the middle of the internodes to avoid leaf vascular traces found near the nodes (Figure 2). The sections should be thin enough and consistent enough to fit under a coverslip (< 0.3 mm) while in water. Cut the stem in a forward and downward motion exposing the tissue to clean blade areas preventing further damage (Figure 3). The blade will dull within roughly 30 cross sections and should be rotated or disposed of. Dull blades will compress the stems and pull the helical thickenings of the secondary cell wall out of the metaxylem.
Note: The cut and damaged cells and those intact but in contact with the water under the coverslip will begin to degrade immediately. It is necessary to image deeper into the tissue than these cells with CSLM. Familiarity with the phenomenon of emission spectra shifting from far red plastid autofluorescence to green is best characterized with wild type plant material. The degradation of chlorophylls from damage causes the spectra to move from far red to green, this may result in autofluorescence being mistaken for GFP.
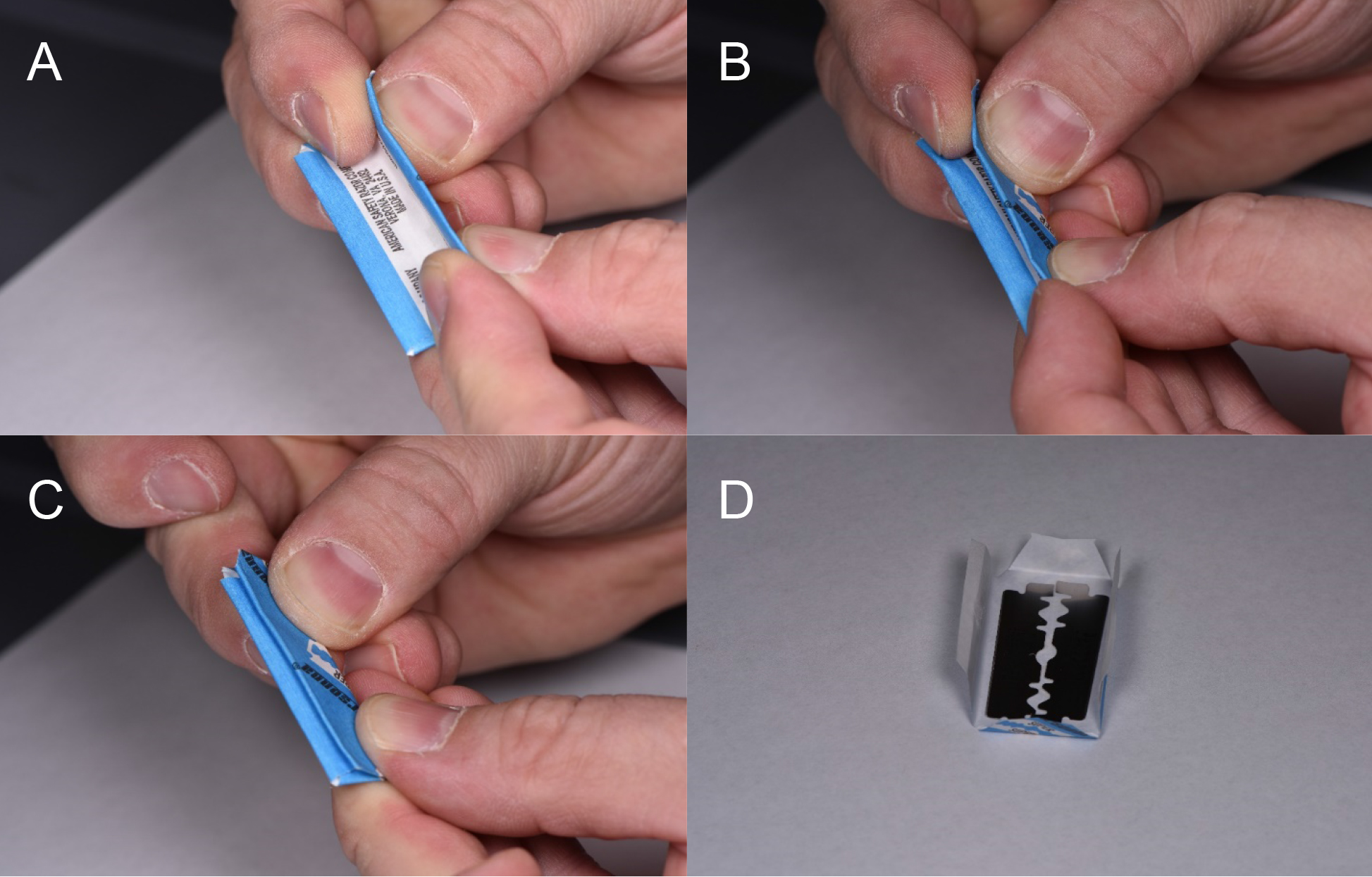
Figure 1. Method for using double edged razor blades. A. Grasp the blade so that the sharp edges are not pressured by the fingers. B. The index fingers are retracted while slow and constant pressure is used to bend the blade in half. C. As the two outer edges come closer, the index fingers and thumbs are placed near the folded side and pressure there will cause the blade to snap into two separate blades. D. Unwrapping the blade is done carefully again to prevent cuts through the paper. Each half of the blade may be used for dissection.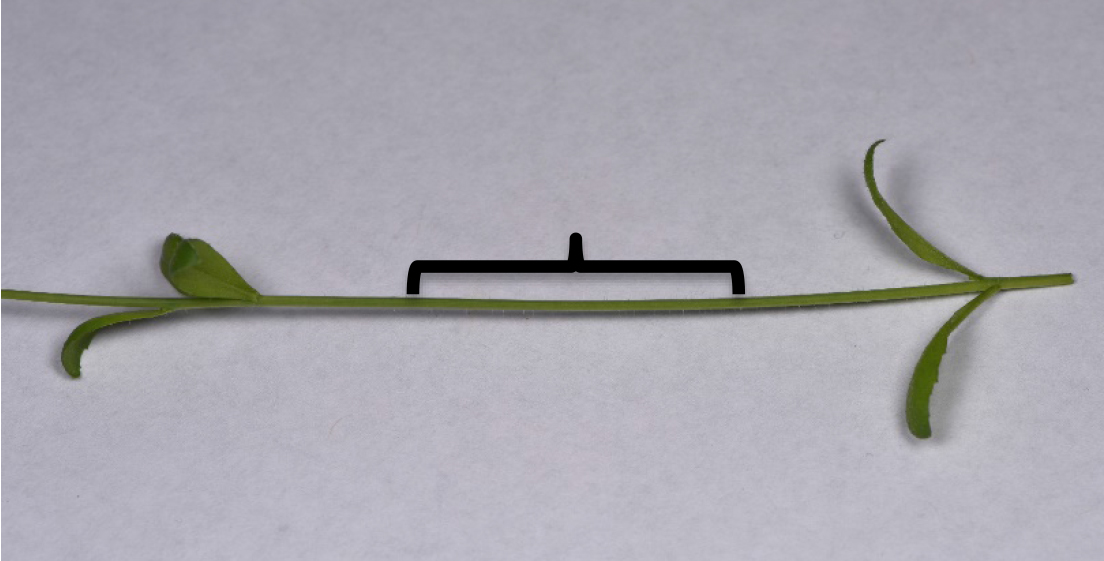
Figure 2. Arabidopsis thaliana flowering stem used for analysis. The internode region is where cross sections should be made (bracket).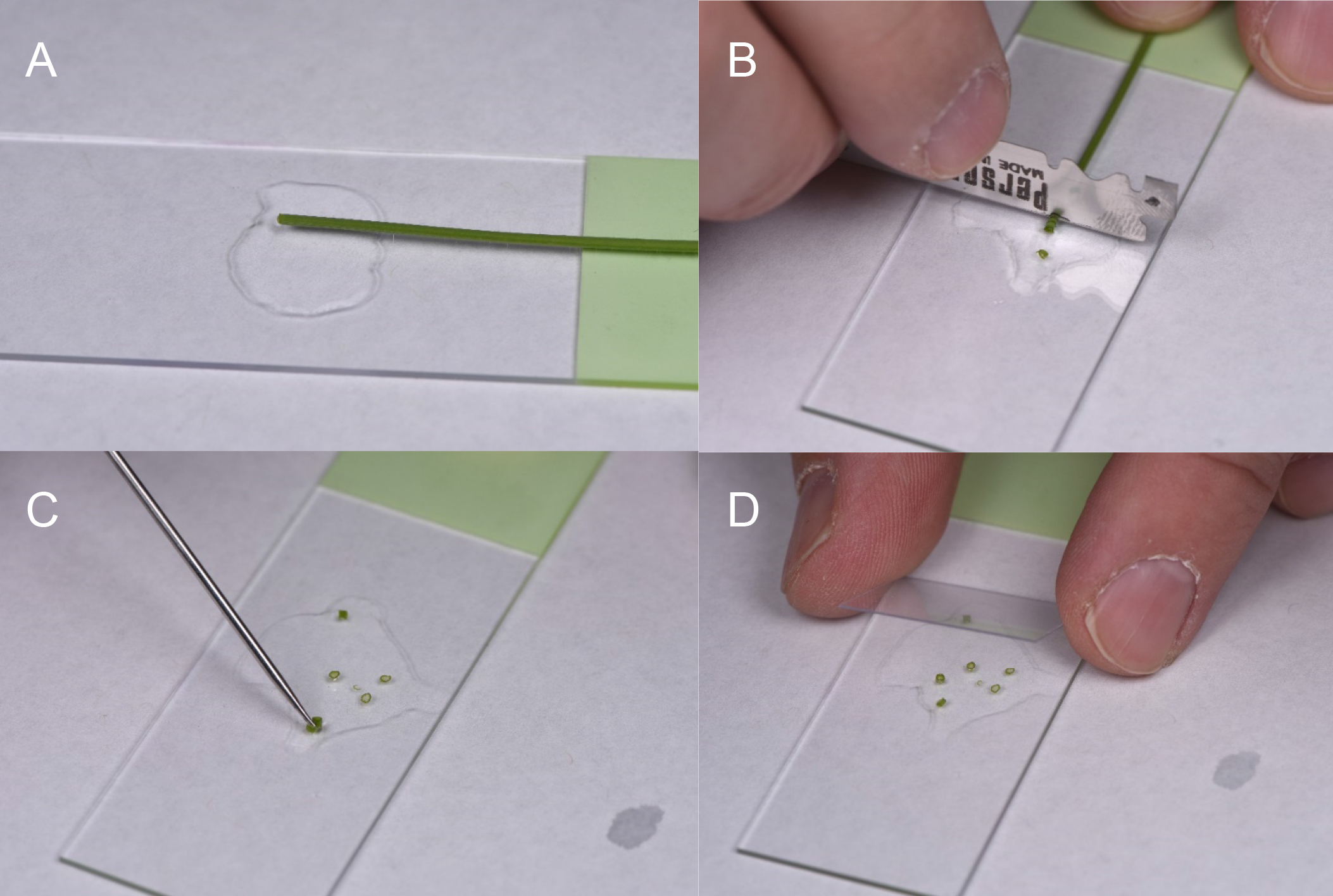
Figure 3. Method for cutting and mounting Arabidopsis stem cross sections. A. The stem with the unneeded portions removed is laid flat on the slide with water. B. Sections are cut as thin as possible in a forward and downward motion. The blade may be rotated to expose a sharp edge, or the other half may be used. C. While cutting, the sections may stack, this is remedied by gentle manipulation with a dissecting needle or probe or insect pin. D. Once all sections are separated, a cover slip is placed on the samples. Uniform sections are important for level cover slip placement. Water may be added or wicked off to keep the sections in water, but without movement.
- GFP or YFP when viewed through the oculars with a broad emission filter coupled with a 488 nm LED will not only allow visualization of GFP or YFP (with less excitation efficiency) but also the plastids. This full spectrum visualization allows the user to differentiate damaged areas where plastids shift from red emission to orange. Furthermore in variegated tissues, ocular observation may be the only method of differentiating low GFP or YFP and damaged/stressed areas. Ocular observations have the benefit of much deeper optical sections, providing context for localization. In this case, 10x and 20x visualization provided clues to pursue vascular localization.
- Preparation of enzymatically digested plant tissues and visualization
Partial enzymatic digestion of A. thaliana leaves for visualization of fluorescence in plant vasculature. Enzymatic digestion is done by modifying the protocol of Yoo et al. (2007) used for Protoplast Isolation Procedure.- Cut the leaves so most of the lamina is trimmed off from the midrib and veins and subjected to enzyme digestion.
Note: A six to seven weeks old plant is normally used for this experiment. - Place the plant material to be digested in a Petri dish containing the enzyme solution and put it in a vacuum desiccator and vacuum infiltrate for 15 min.
Note: A 5-10 ml cellulase/macerozyme solution (see Recipes) is enough for a flowering A. thaliana plant. - Take out the Petri dish from vacuum and continue digestion at RT for 2 h with gentle shaking on a platform shaker, between 30-50 RPM.
- The proper digestion time is near when the epidermis is digested away, and mesophyll cells begin sloughing away. The slurry generated to visualize vascular parenchyma with intact leaf or is easily transferred with a transfer pipette or fine tip forceps as the samples are not allowed to fully digest to the protoplast stage. The sample can be transferred to a Petri dish containing 10 ml of the WI buffer. On a compound microscope, if the vasculature can be seen, the sample is ready for confocal analysis. Vasculature is easily identified by the helical thickenings in the xylem with transmitted light at 10x or 20x. Digestion time will vary with the hardiness of the plant material.
- Mount samples on slides with coverslips for confocal microscopy. The incubation solution is used to mount the sample, as it will support the cells during imaging.
- GFP or YFP can be visualized with RFP with relative ease with the correct filter sets on the CSLM, although not simultaneously. Sequential or simultaneous acquisitions is dictated by the fluorescent protein used and the relative strength compared to various sources of autofluorescence. It is crucial to compare CSLM settings to the wild type material at the same settings used to detect the fluorescent proteins. When expression of the fluorescent protein(s) is low, it is suggested to check and recheck against negative controls.
- Plastid suborganellar localization often requires practices outside of routine confocal imaging. Operators should be aware of data clipping from under and overexposure of the detectors. Underexposed pixels may imply lost data. Overexposed pixels are data lost, and there is concern regarding overloading of the detectors. NIS-Elements has an indicator display for under and overexposed pixels. When expression levels are low or near the same signal levels as autofluorescence, it is suggested that the look up table (LUT) be manipulated to increase the brightness on the display screen after image acquisition. Images acquired in the same way between the negative and putative positives should be viewed with these same artificial increases in signal. Increasing the signal to the detectors will only increase the artifacts and noise in the images. Chlorophyll autofluorescence, detected in the 650-720 nm range at 60x or higher will overlap autofluorescence in other detection channels. Signal not colocalized with the chlorophyll autofluorescence may indicate positive fluorophore signal. It is crucial to repeat the experiment and continue to compare the images between the negative and putative positives.
Nikon A1 CSLM notes: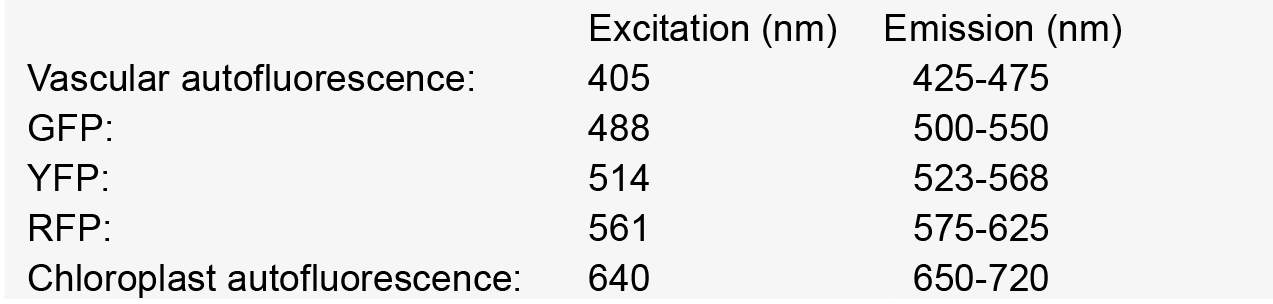
- Cut the leaves so most of the lamina is trimmed off from the midrib and veins and subjected to enzyme digestion.
Data analysis
Factors that affect sample observations:
- Transgene construct features influence microscopy outcomes
Promoter selection can influence signal strength significantly in our experiments. Consequently, we have combined both transient, high level expression experiments with native promoter, stable transformation experiments for any constructs to be tested (Figure 4). For proteins targeted to subcellular organelles, it appears that truncation of the full-length gene can sometimes produce unreliable results. Figure 5 shows that the MSH1 protein, which targets to the nucleoid subcompartment of the plastid, can be found localized to the nucleoid only when at least domains I–III of the protein coding region are included in the analysis (Virdi et al., 2016).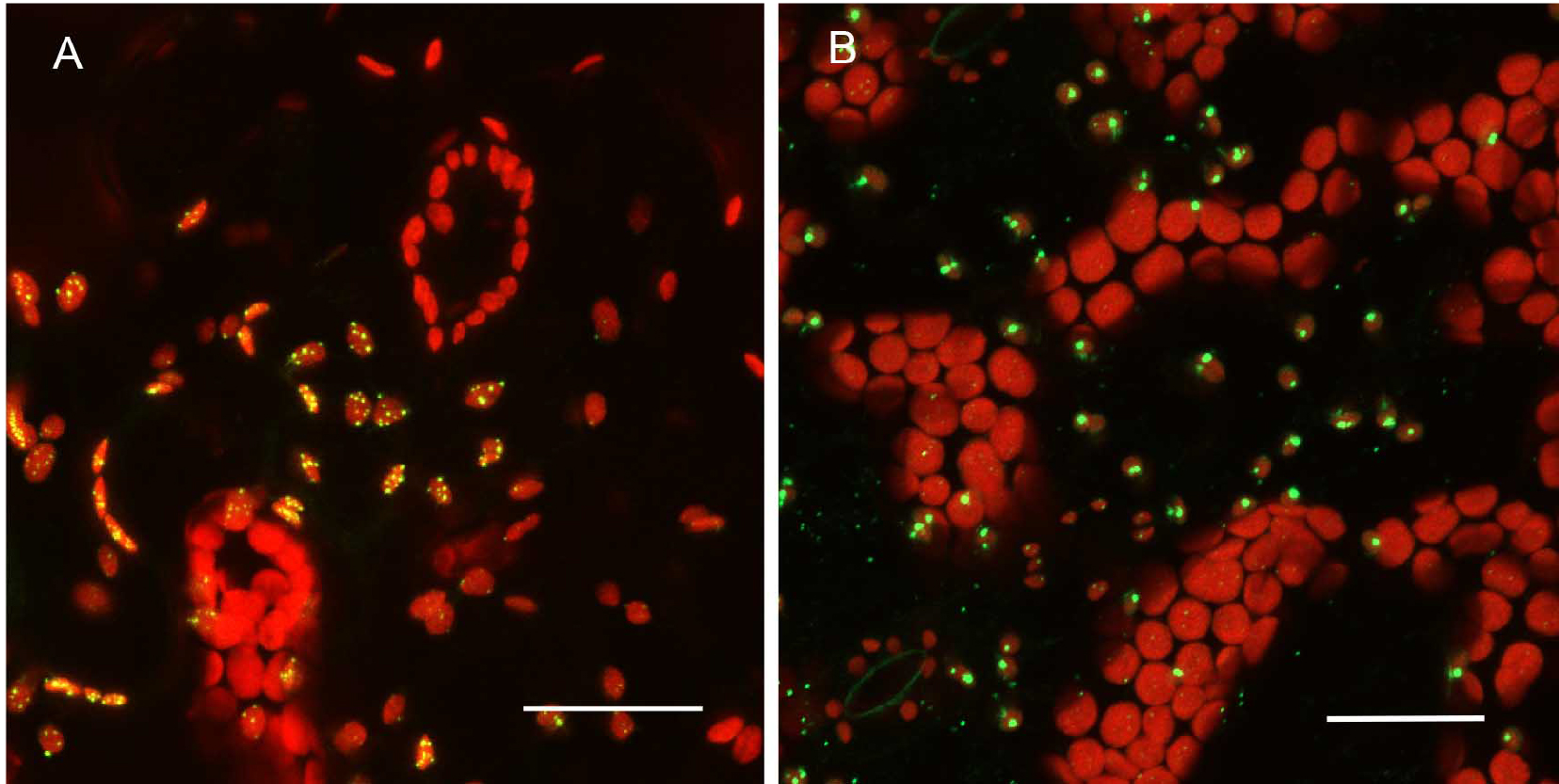
Figure 4. Expression pattern in leaves and level of expression vary depending on the promoter used. A. pCAMBIA1302C::35S::MSH1::GFP transient expression in tobacco. There are more punctate GFP expression signals when the gene construct is driven by the CaMV 35S promoter. B. pCAMBIA1302C (-35S)::NativePro::MSH1::GFP stable expression in Arabidopsis cells. The native promoter resulted in fewer punctate GFP signals (as seen by number of green dots per plastid). All scale bars are 25 µm. GFP pseudocolored - green, chloroplast autofluorescence pseudocolored - red, maximum intensity projections.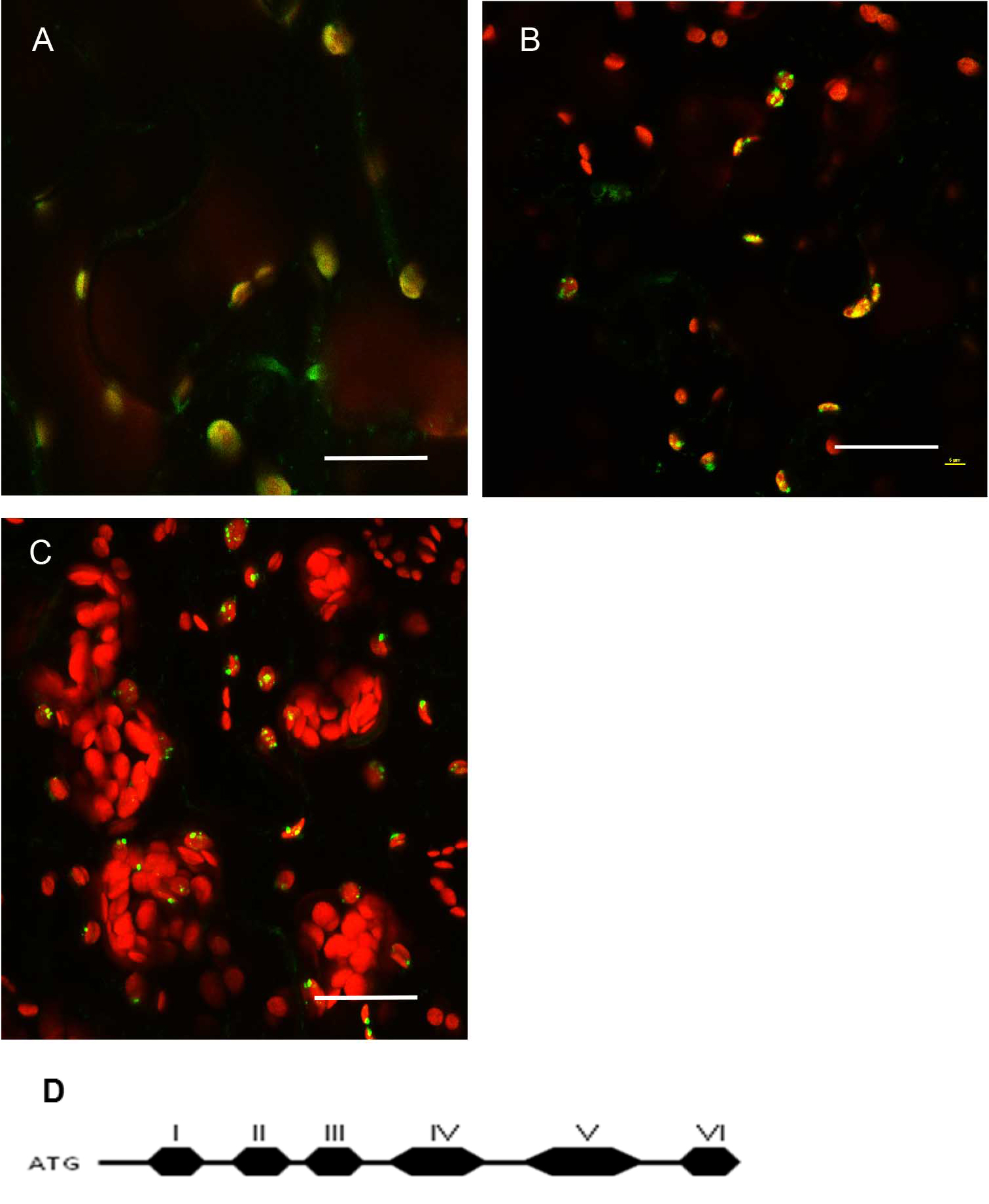
Figure 5. Protein localization pattern can vary depending on truncations in Arabidopsis thaliana Col-0 leaf tissue. A. Native promoter::MSH1(ATG to Domain I)::GFP; domain I alone gives a diffused signal in the plastids. Single frame image. B. Native promoter::MSH1(ATG to Domain III)::GFP. Single frame image. C. Native promoter::MSH1(ATG to Domain V)::GFP. MSH1 ATG start to domain III is necessary to give the punctate localization. Maximum intensity projection. GFP pseudocolored green, chloroplast autofluorescence pseudocolored red for all images. All scale bars are 25 µm. D. Schematic diagram of the different domains of MSH1 from Arabidopsis thaliana used for the truncation studies (Virdi et al., 2016). - Tissue preparation can also influence resolution in a confocal microscopy study
Plants stably transformed with a full-length gene construct under control of its native promoter offer the opportunity to document spatial and temporal expression behaviors. Figure 6 shows the result of partial tissue digestion in revealing the localization of the MSH1 protein within specialized plastids of the vascular parenchyma tissues. These observations are confirmed, in Figure 7, by stem cross-sections that show MSH1-GFP signal within the vascular bundle tissues. However, it is essential that the tissues assayed are not derived from a stressed plant. Figure 8 shows that artifactual fluorescence in a plant under stress can be confused with RFP signal.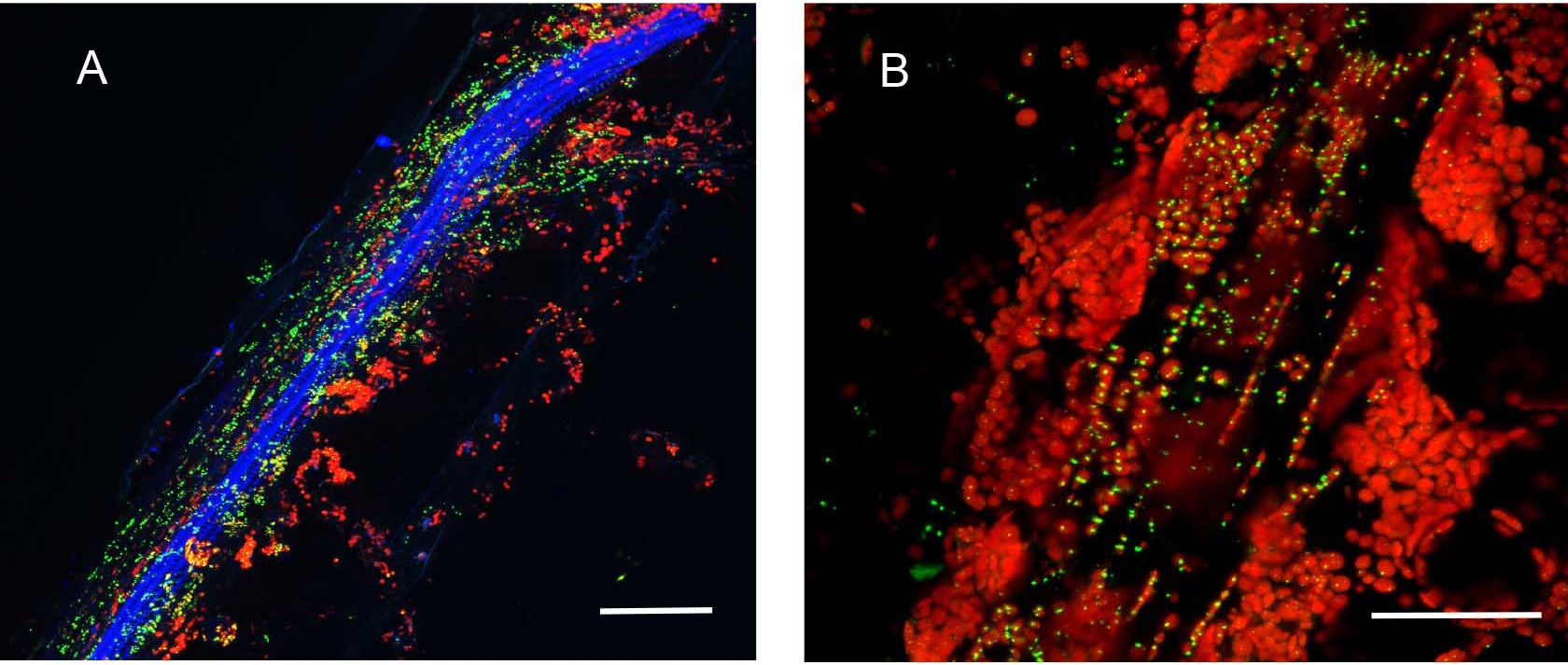
Figure 6. Partial enzymatic digestion of A. thaliana leaf midrib and side veins to study sub-cellular localization of fluorescence. Native Pro::MSH1::GFP stable transformed A. thaliana Col-0 leave digested with cellulose and macerozyme as described above. MSH1::GFP expression in specialized plastids can be seen concentrated around phloem and xylem but not in the mesophylls. All scale bars are 25 µm. A. Minor leaf vein. GFP pseudocolored - green, chloroplast autofluorescence pseudocolored - red, xylem autofluorescence pseudocolored - blue. Maximum intensity projection. B. Midrib of leaf. GFP pseudocolored - green, chloroplast autofluorescence pseudocolored - red.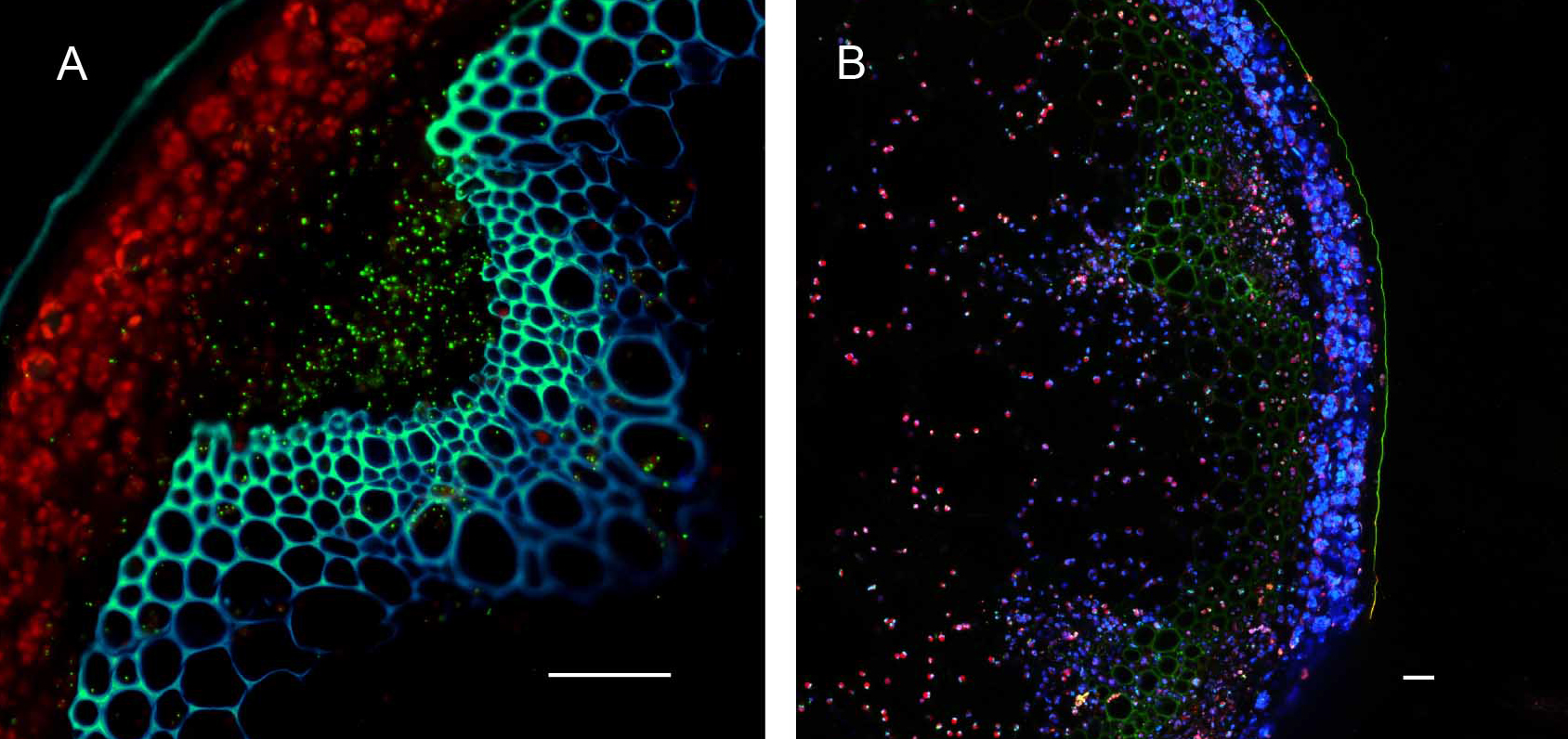
Figure 7. Hand sectioning technique to visualize tissue specificity of proteins. A. Cross section of native promoter::MSH1::GFP stable transformed floral stems hand sectioned as described in Materials and Methods. MSH1::GFP expression localizes to the vascular system. GFP pseudocolored green, chloroplast autofluorescence pseudocolored red, xylem autofluorescence pseudocolored blue with crosstalk in the GFP channel. B. Cross section of a dually transformed A. thaliana with MSH1::GFP and PPD3::RFP (AT1G76450); co-localization of two proteins in the same tissue and plastid type. GFP pseudocolored - green, RFP pseudocolored - red, chloroplast autofluorescence pseudocolored - blue. Single frame images and all scale bars are 25 µm.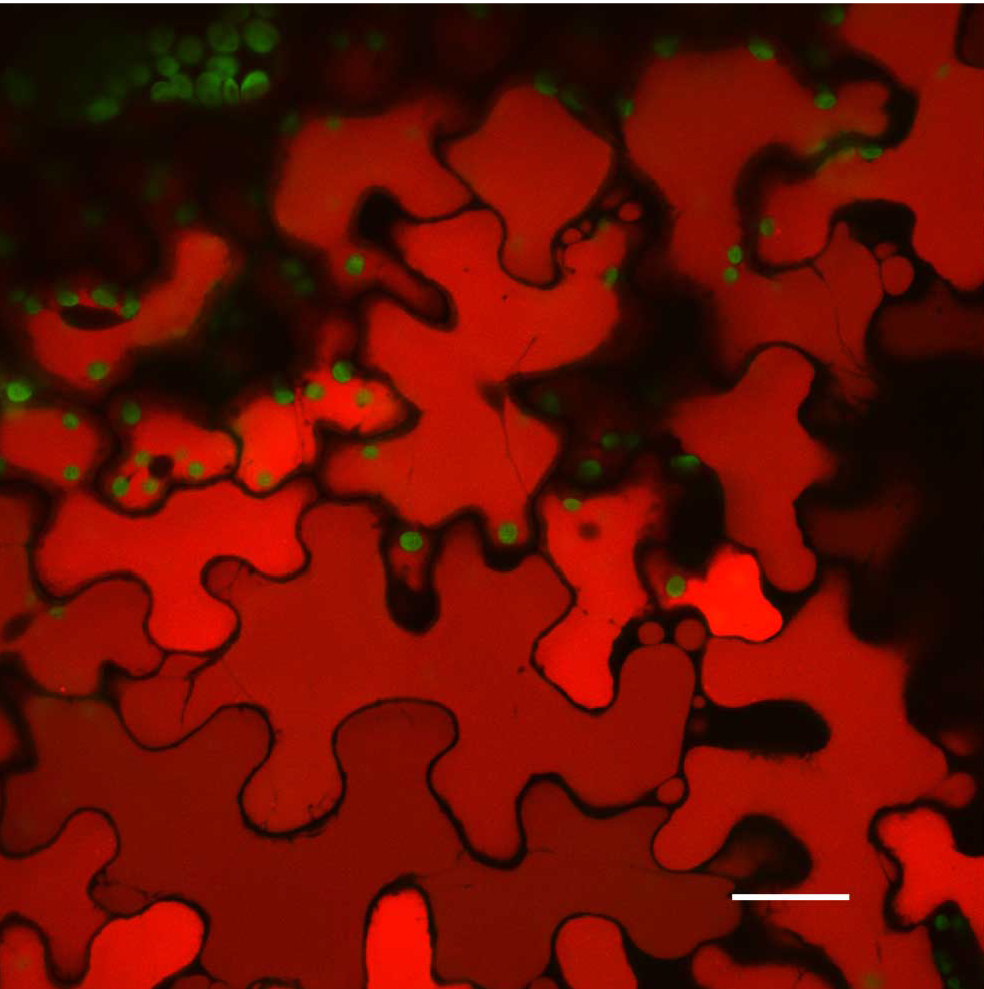
Figure 8. Artifacts that users need to be aware of in Confocal Microscopy. A stressed A. thaliana Col-0 leaf vacuoles. Scale bar is 25 µm. Chloroplast autofluorescence pseudocolored - green, vacuole autofluorescence pseudocolored - red. Maximum intensity projection.
Recipes
- Induction medium (1 L)
10.5 g K2HPO4
4.5 g KH2PO4
1 g (NH4)2SO4
0.5 g sodium citrate dihydrate
1 ml MgSO4 (1 M stock solution)
2 g glucose
5 ml glycerol
10 mM MES
Adjust pH to 5.6 with KOH or NaOH and autoclave at 115 °C for 20 min
Cool down medium and add antibiotics and 50 μg/ml acetosyringone - Infiltration medium
Half strength MS medium basal salts
10 mM MES
Adjust pH to 5.6 with KOH
Add 150 μg/ml acetosyringone before use - Cellulase/macerozyme solution
1.5% cellulase ‘onozuka’ R-10 (0.15 g/10 ml)
0.4% macerozyme R-10 (Yakult Honsha, Tokyo, Japan) (0.04 g/10 ml)
0.4 M mannitol (0.8 M mannitol stock)
20 mM KCl (2 M KCl stock)
20 mM MES, pH 5.7 (0.2 M MES stock)
Heat the enzyme solution in a 55 °C water bath for 10 min in 15 ml centrifuge tube (stirring not needed)
Cool it to room temperature
Add 10 mM CaCl2 (1 M CaCl2 stock); 0.1% BSA (optional) (10% stock, sterile)
Pass the enzyme through a 0.45 μm filter - Washing and incubation solution (WI)
0.5 M mannitol
4 mM MES, pH 5.7
20 mM KCl
Acknowledgments
We wish to thank Dr. Kamaldeep Virdi and Sunil Kumar for supplying images from their research in Arabidopsis for demonstration purposes. We also thank Cecil Renfro for her assistance with photography. We gratefully acknowledge support from the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences of DOE (DE-FG02-10ER16189) to S.M. for this work.
References
- Baginsky, S. and Gruissem, W. (2004). Chloroplast proteomics: potentials and challenges. J Exp Bot 55(400): 1213–1220.
- Van den Ackerveken, G., Marois, E. and Bonas, U. (1996). Recognition of the bacterial avirulence protein AvrBs3 occurs inside the host plant cell. Cell 87(7):1307–1316.
- Virdi, K. S, Wamboldt, Y., Yu, J., Laurie, J. D., Kumar, S., Kundariya, H., Xu, Y., Elowsky, C., Basset, G., Bricker, T., Leubker, S., Keren, I. and Mackenzie, S. A. (2016). MSH1 is a plant organellar DNA binding and thylakoid protein under precise spatial regulation to alter development. Mol Plant 9: 245–60.
- Xu, Y. Z., Arrieta-Montiel, M. P., Virdi, K. S., de Paula, W. B., Widhalm, J. R., Basset, G. J., Davila, J. I., Elthon, T. E., Elowsky, C. G., Sato, S. J., Clemente, T. E. and Mackenzie, S. A. (2011). MutS HOMOLOG1 is a nucleoid protein that alters mitochondrial and plastid properties and plant response to high light. Plant Cell 23: 3428–3441.
- Yoo, S. D., Cho, Y. H. and Sheen, J. (2007). Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2(7): 1565-1572.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Elowsky, C., Wamboldt, Y. and Mackenzie, S. (2017). Laser Scanning Confocal Microcopy for Arabidopsis Epidermal, Mesophyll, and Vascular Parenchyma Cells. Bio-protocol 7(5): e2150. DOI: 10.21769/BioProtoc.2150.
Category
Plant Science > Plant cell biology > Cell imaging
Cell Biology > Cell imaging > Confocal microscopy
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










