- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Miniature External Sapflow Gauges and the Heat Ratio Method for Quantifying Plant Water Loss
Published: Vol 7, Iss 3, Feb 5, 2017 DOI: 10.21769/BioProtoc.2121 Views: 9714
Reviewed by: Scott A M McAdamEunsook ParkYuko Kurita
Abstract
External sapflow sensors are a useful tool in plant ecology and physiology for monitoring water movement within small stems or other small plant organs. These gauges make use of heat as a tracer of water movement through the stem and can be applied in both a laboratory and a field setting to generate data of relatively high temporal resolution. Typical outputs of these data include monitoring plant water use on a diurnal time scale or over a season (e.g., in response to increasing water deficit during drought) to gain insight into plant physiological strategies. This protocol describes how to construct the gauges, how best to install them and some expected data outputs.
Keywords: SapflowBackground
Sapflow technology is a tool in plant ecology that uses heat as a proxy of water flow within stems or other plant organs. Although a variety of sapflow methods have been developed (see review by McElrone and Bleby, 2011), external miniature sapflow gauges make use of the heat ratio method (HRM) (Burgess et al., 2001) for estimating plant sap velocity.
The HRM relies upon two thermocouples evenly spaced either side of a heating element along the same axis as the flow of sap (Burgess et al., 2001). (Note: Generally, water moves up through a plant from the roots towards the leaves, where it is lost through stomatal pores during evapotranspiration [E].) Marshall (1958) showed that for low rates of flow the ratio of the downstream temperature differential, T1, to the upstream temperature differential, T2, provides an accurate estimation of the heat pulse velocity, vh:
vh = α/x ln (δ T1/δ T2), in cm s-1 (Eq. 1)
Where,
α is the thermal diffusivity (cm2 s-1),
x is the distance above or below the heating element (cm).
When there is no sapflow the ratio of δ T1 to δ T2 is equal to one and thus the logarithm of the ratio of the two temperature differentials is zero. When sapflow occurs, the ratio of δ T1 to δ T2 is less than or greater than 1, with values above 1 measuring flow towards the leaves and values below 1 indicating reverse flow towards the roots.
The thermal diffusivity, α, can be estimated by recording the temperature profile of one of the thermocouples following a heat pulse under conditions of zero flow (Clearwater et al., 2009). It is proportional to the amount of time it takes for the thermocouple to reach a maximum temperature, tm, after a heat pulse:
α = x2/4 tm, in cm2 s-1(Eq. 2)
For miniature external gauges, α is a property of the gauge material and the properties of the stem with which it is in contact. A significant proportion of heat is propagated through the gauge block and α varies little between individuals of a species (Clearwater et al., 2009). α can be estimated from Eq. 2 by installing gauges on excised stems of study species and recording heat pulses with no imposed xylem flow. Thereafter α can be assumed to be constant for a species and applied in Eq. 1 for other individuals of the same species. Vandegehuchte and Steppe (2012) showed recently that thermal diffusivity, α, of woody stems may vary throughout a growing season, which affects calculations of vh. An improved estimate of α and how it varies throughout a growing season is desirable and may improve the fit between vh and transpiration (E).
Figure 1. Miniature external sapflow gauge with 10 m lead cable connected to a small branch of a potted Umbellularia californica plant and a Campbell Scientific CR10X data logger
Materials and Reagents
Note: Most of these materials can be obtained by ordering online from omega.com or by visiting local electrical supply stores.
- 2 x 4 cm of 0.15 mm (35 AWG) copper and teflon or plastic coated constantan wire
- 47 ohm pad resistor
- Fast-setting silicone
- 15 m of 4-core 0.51 mm (24 AWG) cable
- 15 m of 0.8 mm (20 AWG) constantan wire
- 10 cm of 2 mm heat shrink wire wrap tubing
- 15 cm of 1.2 cm heat shrink wire wrap tubing
- 62 Ohm resistor
- Circuit board builder
- Pluggable terminal blocks (compatible with circuit board)
- Parafilm (Bemis Company, Inc., Neenah, WI, USA)
- Polystyrene block approximately 3 x 15 x 9 cm (but could be modified depending on the size of the branch)
- Durable and reflective foil (e.g., roof insulation foil)
Equipment
- Soldering iron
- Solder
- Single edged razor blade
- Multimeter
- Data logger (e.g., CR1000 or CR10X Campbell loggers, Campbell Scientific, model: CR1000 or CR10X)
Software
- ImageJ (ImageJ 1.47v, National Institutes of Health, USA)
Procedure
- Gauge design and setup
Sapflow gauges were constructed following the design presented by Clearwater et al., (2009) and Skelton et al., (2013). The miniature external gauges consist of two thermocouple junctions spaced equidistant above and below a heater element (Figures 2 and 3). The gauges can be connected to Campbell Scientific loggers (Campbell Scientific Inc., Logan, UT, USA) with up to 10 m leads.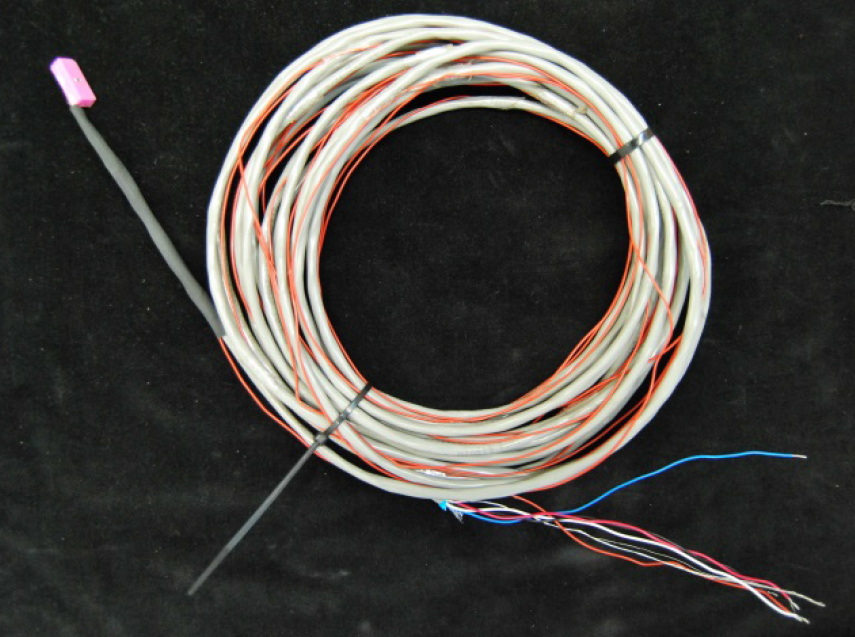
Figure 2. Miniature external sapflow gauge with 10 m lead cable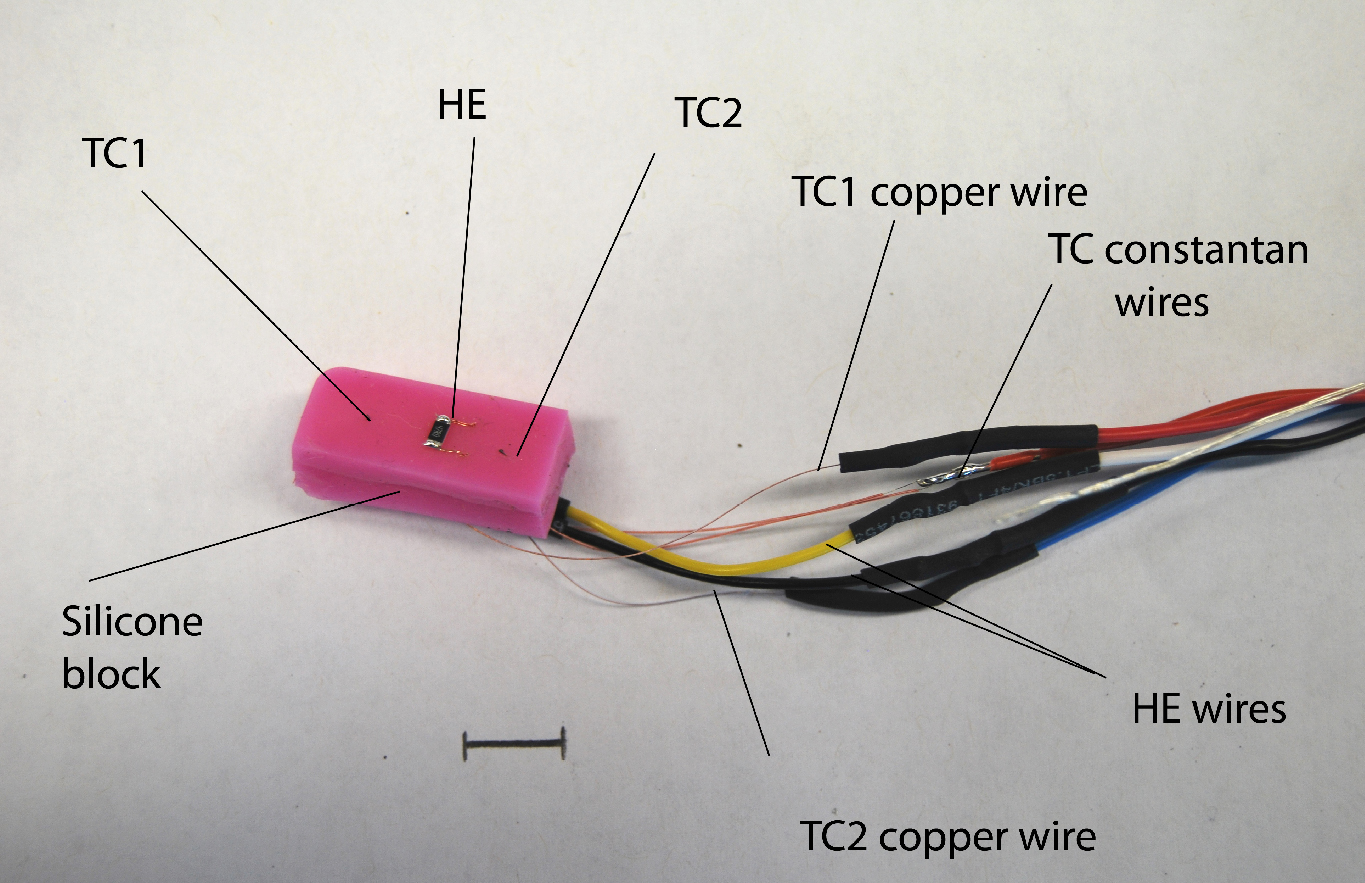
Figure 3. Gauge head showing the silicone backing block with the heating element (HE) between two copper-constantan thermocouples (TC1 & TC2) and the wires. Scale bar = 1 cm. - Gauge construction
- Gauge thermocouples
- Cut two 4 cm long pieces of 0.15 mm (36 AWG) copper and constantan wire. Remove about 2-3 mm of the enamel coating from the ends of the copper wire and plastic coating from the ends of the constantan wire using a razor blade.
- Solder each copper-constantan pair at one end to make a thermocouple junction.
Note: Although you want the mass of the junction to be as small as possible, there is a trade-off between ensuring contact with the plant material and the mass of the junction. Play around with different size junctions to see which works for your species/system. (e.g., if you are working on branches > 0.5 cm diameter you may be able to use slightly larger junctions.)
- Cut two 4 cm long pieces of 0.15 mm (36 AWG) copper and constantan wire. Remove about 2-3 mm of the enamel coating from the ends of the copper wire and plastic coating from the ends of the constantan wire using a razor blade.
- Gauge heater element
- Solder two 4 cm long copper cables to a 47 ohm pad resistor.
Note: It may be necessary to strip back about 4 mm of the copper cable to isolate a single wire strand and connect this to the pad resistor. I have also tried to thread the two copper cables through two holes in the gauge backing and then solder them to the pad resistor. This makes it easier to anchor the pad resistor in position in the middle of the gauge (see Figure 2). Finally, some gauge moulds have a small indentation/depression into which the resister pad can nestle. If you don’t have one, it might be useful to make a small indentation in the silicone backing to ensure that the heating element fits snugly into the mould.
- Solder two 4 cm long copper cables to a 47 ohm pad resistor.
- Gauge head
- Create a 10 x 20 x 6 mm gauge mould using fast-setting silicone or any other non-conductive material (e.g., cork).
- Thread the two thermocouple junctions through the silicon backing. It is important to ensure that the thermocouples are equidistant at a distance of ~0.6 cm from the heater element.
- The silicone mould should have six wires extending out the back. Twist the ends of the constantan wires together, and solder them, to ensure that the two TC junctions share a common (constantan) ground.
- Create a 10 x 20 x 6 mm gauge mould using fast-setting silicone or any other non-conductive material (e.g., cork).
- Lead wires
- The lead wires extend from the sensors to the data logger. For the leads, use 4-core 0.51 mm (24 AWG) cable and attach a 0.8 mm (20 AWG) constantan wire to the outside. Cut both 4-core and constantan wires to up to 10 m lengths, and tape the two wires together about every 1 m.
- Strip one end of the lead wire a little way back (about 20-30 cm), exposing the four (insulated) cables. Of the four exposed copper cables, the two TC copper wires will be connected to the ‘High’ ends of two Differential channels on a logger (e.g., 1 H and 2 H on a Campbell logger) or a multiplexer and the other two wires to the ground and 12 V of the logger (see below).
- Strip about 5-10 cm of the outer insulation from the other end of the lead wire. These wires will be connected to the gauge heads (Figure 3).
- The lead wires extend from the sensors to the data logger. For the leads, use 4-core 0.51 mm (24 AWG) cable and attach a 0.8 mm (20 AWG) constantan wire to the outside. Cut both 4-core and constantan wires to up to 10 m lengths, and tape the two wires together about every 1 m.
- Connecting the gauge to the lead wire
- Slide the 1.2 cm heat shrink wire wrap tubing onto the lead cable prior to connecting the gauge head to the lead cable.
- Cut 2 cm lengths of 0.2 mm heat-shrink wire wrap tubing and push them onto each of the five lead cable wires prior to connecting these to the five wires extending from the silicon mould (see Figure 3).
- Solder two of the stranded cores in the wire to the copper ends of the TC junctions. Decide on a convention for the wiring (e.g., use the red core wire for the downstream TC and the blue core wire for the upstream TC).
- Solder the constantan in the lead wire to the joined constantan wire from the two TCs.
- Check the junctions at the base of the hookup wire with a multimeter.
- Shrink the heat shrink wire wrap tubing by heating gently, ensuring that the protective covering is snug and thus provides good insulation for each of the junctions.
- Slide the 1.2 cm heat shrink wire wrap tubing onto the lead cable prior to connecting the gauge head to the lead cable.
- Heater panel
- The heater panel is required to split the current from the 12 V port on the datalogger to up to 16 parallel ports (if using an AM16/32 relay multiplexer, Campbell Scientific Inc., Logan, UT, USA), each with a 62 Ohm resistor in series. Each sensor should be wired into its own port on the heater panel.
- Solder two pluggable terminal blocks and the 62 Ohm resistor to the circuit board (Figure 4).
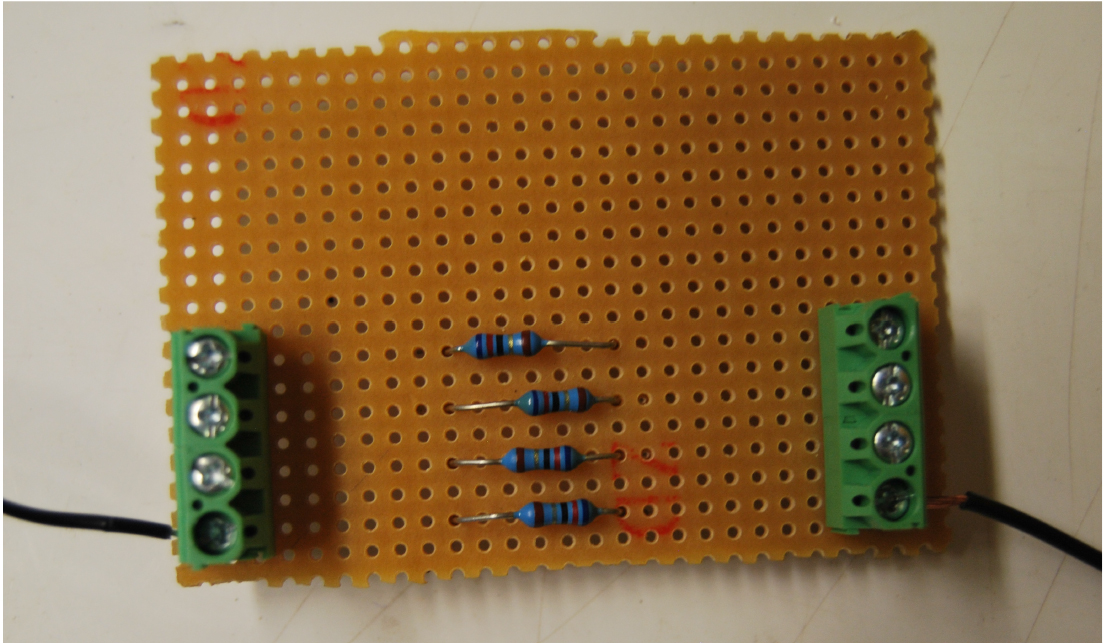
Figure 4. The heater panel consisting of pluggable terminal blocks (green), a 62 Ohm resistor (blue) connected on a circuit board
- The heater panel is required to split the current from the 12 V port on the datalogger to up to 16 parallel ports (if using an AM16/32 relay multiplexer, Campbell Scientific Inc., Logan, UT, USA), each with a 62 Ohm resistor in series. Each sensor should be wired into its own port on the heater panel.
- Gauge thermocouples
- Connecting the gauges to the logger
- Connect one end of the gauge heater element wire (positive) to the heater panel (i.e., in series with the 62 Ohm resistor) and the other end (negative) to the Ground channel on the datalogger (e.g., CR1000, Campbell Scientific Inc.). Connect the heater panel to the 12 V channel of the datalogger.
- Connect the copper ends of the two TCs to separate H channels of a datalogger or a multiplexer connected to a datalogger (e.g., AM16/32 relay, Campbell Scientific Inc.), depending on the amount of gauges required.
- Connect the single constantan wire to the L channel of the datalogger/multiplexer and extend a linking constantan cable to the second L channel.
- Use the datalogger to fire a 4 sec heat pulse through the heater element every 30 min. The length of the heat pulse and the frequency of sampling may be varied depending on individual study species and data requirements.
- Record an average of the TC temperatures over ~5 sec immediately before the release of the heat pulse and use this as a reference temperature.
- Record the TC temperature over a fixed period (usually from 55 to 75 sec) after the heat pulse. The fixed period will vary between species and should be chosen to cover the period of greatest stability in temperature differentials, taking into consideration that earlier measurements are prone to overestimating heat pulse velocity (Burgess et al., 2001).
- Use the average ratio of the temperature differentials to calculate heat pulse velocity, vh(using equation 1).
- Connect one end of the gauge heater element wire (positive) to the heater panel (i.e., in series with the 62 Ohm resistor) and the other end (negative) to the Ground channel on the datalogger (e.g., CR1000, Campbell Scientific Inc.). Connect the heater panel to the 12 V channel of the datalogger.
- Installing the gauges
- The gauges should be tightly connected to the stem or plant organ using a waterproof, cohesive film, such as Parafilm (Bemis Company, Inc.), which can then be insulated with duct tape (Figure 5).
- The gauges should be insulated with relatively light materials (e.g., polystyrene blocks, Figure 6) and covered with reflective foil to ensure minimal distortion of the heat signal (Figure 7).
Note: I usually use 3 x 15 x 9 cm polystyrene blocks (as shown in Figure 6). Alternatively, you could also use expanding polyurethane or ‘plumbers foam’ with a polystyrene or plastic cup.
Figure 5. External miniature sapflow gauge connected to a plant shoot and held in place by black insulation tape. The gauges should be tightly, but carefully connected to the plant stem using Parafilm and duct tape.
Figure 6. Polystyrene insulation blocks positioned around the external sapflow gauges. The gauges should be insulated using light materials, such as two blocks of polystyrene (one of which has a groove carved into it), to insulate them from external changes in temperature that may otherwise affect the heat ratio values.
Figure 7. Completed insulation of the external sapflow gauge. Gauges installed on a small branch of a potted Acacia mearnsii were insulated with polystyrene and covered in reflective foil.
- The gauges should be tightly connected to the stem or plant organ using a waterproof, cohesive film, such as Parafilm (Bemis Company, Inc.), which can then be insulated with duct tape (Figure 5).
- Methods for converting heat pulse velocity (vh) to transpiration
Measured vh can be converted to more commonly used measures, such as transpiration (E), through the use of an empirical multiplier (Cohen and Fuchs, 1989), which can be estimated in a variety of ways.
A reliable empirical multiplier, termed msap, can be gained from the slope of the relationship between vh, and the rate of water loss measured gravimetrically and expressed per stem cross-sectional area, J (in mmol m-2 stem area s-1):
J/vh = msap (Eq. 3)
vh can then be converted to leaf level transpiration rate (E in mmol m-2 leaf area s-1) by dividing by leaf area to stem area ratio (Al:As).
E = (vh x msap)/(Al/As) (Eq. 4)
Leaf area can be quantified by harvesting either all of the leaves or a subsample of the leaves, scanning them with a flatbed scanner and analyzing for leaf area (cm2) using ImageJ (ImageJ 1.47v, National Institutes of Health, USA). Stem area can be quantified by calculating the cross-sectional area of the conducting xylem tissue. This can be done by harvesting the branch, removing the non-conductive tissue (e.g., pith and bark) and the phloem if possible or by imposing flow of a dye solution through the stem. Unfortunately, this approach of quantifying E from sap velocity requires that instrumented stems be destructively harvested and have a flow imposed through the xylem (Clearwater et al., 2009; Skelton et al., 2013).
Alternatively, vh, can be converted to a transpiration rate using the relationship between sap velocity and E measured using an Infra-Red Gas Analyser (Li-Cor 6400; Li-Cor BioSciences, Lincoln, NE, USA). To do this, leaves from three individuals must be sampled for gas exchange multiple times either on multiple sunny days or through a diurnal period. Gas exchange must be measured under ambient conditions and mean values for each tree for several time points must be compared with vh to ensure good correspondence. For example, the relationship between mean midday E sampled half hourly between 12:00 and 14:00 and mean sapflow-derived vh can be established by sampling over several days through a period of increasing water deficit.
Data analysis
Data outputs are specific to the program that each researcher loads onto the logger. Typical data outputted from the logger include the raw heat ratio traces (see Figure 8a), which in this case were outputted every 15 min. Raw heat ratio traces can be transformed to heat pulse velocity, vh, using equations 1 and 2. Vh values can then be converted to transpiration, E, following the methods outlined in the previous section (Figure 8b) (Skelton et al., 2013). 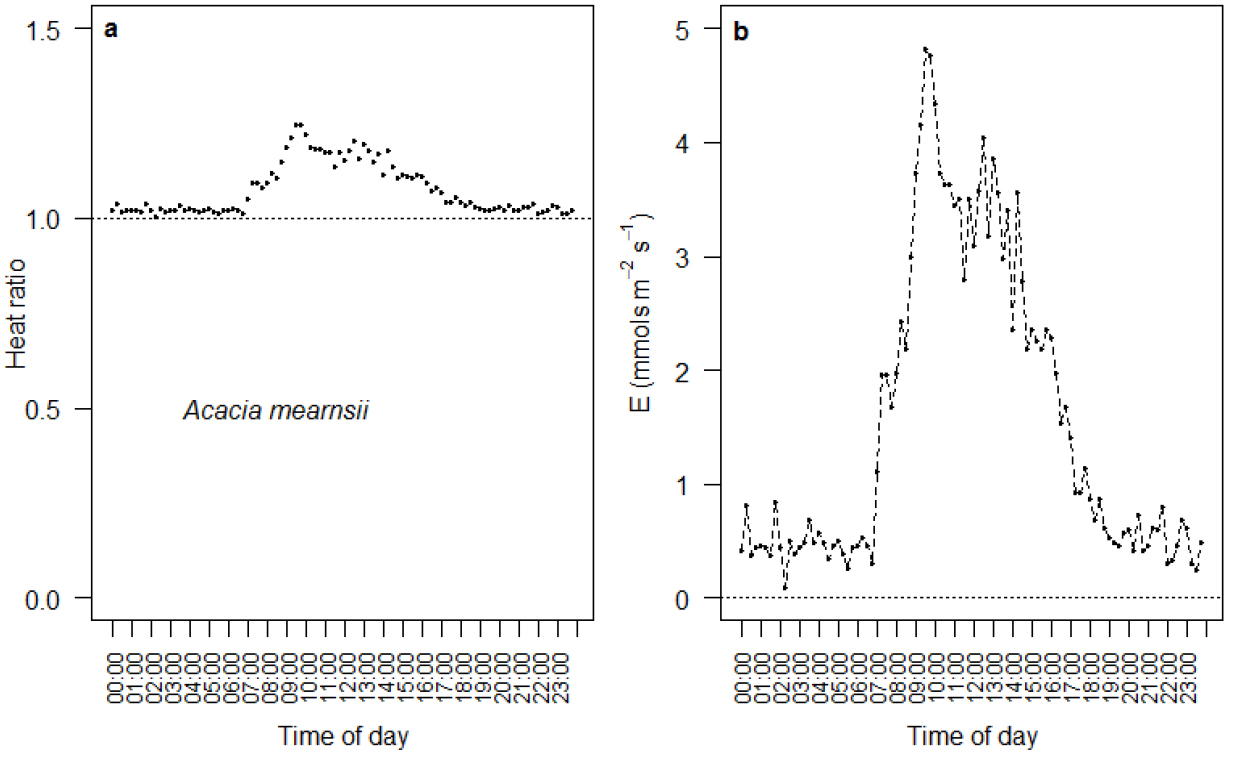
Figure 8. Example of a typical diurnal sapflow trace for Acacia mearnsii, showing the raw heat ratio values (a) and the empirically corrected transpiration (E) values (b). Data were captured every 15 min. Here, we see water use increasing during the morning (from 08:00), declining around midday and then shutting down completely in the late afternoon as the sunlight decreases. These patterns are caused by stomata on the surface of leaves opening and closing.
Acknowledgments
Adam West (University of Cape Town, South Africa), Todd Dawson (University of California, Berkeley, USA), Adam Roddy (Yale University, USA), Michael Clearwater (University of Waikato, New Zealand) and Timothy Brodribb (University of Tasmania, Australia) provided meaningful discussion and contributed enormously to this methodology.
References
- Burgess, S. S., Adams, M. A., Turner, N. C., Beverly, C. R., Ong, C. K., Khan, A. A. and Bleby, T. M. (2001). An improved heat pulse method to measure low and reverse rates of sap flow in woody plants. Tree Physiol 21(9): 589-598.
- Clearwater, M. J., Luo, Z., Mazzeo, M. and Dichio, B. (2009). An external heat pulse method for measurement of sap flow through fruit pedicels, leaf petioles and other small-diameter stems. Plant Cell Environ 32(12): 1652-1663.
- Cohen, Y. and Fuchs, M. (1989). Problems in calibrating the heat pulse method for measuring sap flow in the stem of trees and herbaceous plants. Agronomie 9(4): 321-325.
- Marshall, D. C. (1958). Measurement of sap flow in conifers by heat transport. Plant Physiol 33(6): 385-396.
- McElrone, A. J. and Bleby, T. M. (2011). Sap flow. Prometheus Wiki: 1.
- Skelton, R. P., West, A. G., Dawson, T. E. and Leonard, J. M. (2013). External heat-pulse method allows comparative sapflow measurements in diverse functional types in a mediterranean-type shrubland in South Africa. Funct Plant Biol 40(10): 1076-1087.
- Vandegehuchte, M. W. and Steppe, K. (2012). A triple-probe heat-pulse method for measurement of thermal diffusivity in trees. Agric For Meteorol 160(2): 90-99.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Skelton, R. P. (2017). Miniature External Sapflow Gauges and the Heat Ratio Method for Quantifying Plant Water Loss. Bio-protocol 7(3): e2121. DOI: 10.21769/BioProtoc.2121.
Category
Plant Science > Plant physiology > Transpiration
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











