- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Simultaneous Intranasal/Intravascular Antibody Labeling of CD4+ T Cells in Mouse Lungs
(*contributed equally to this work) Published: Vol 7, Iss 1, Jan 5, 2017 DOI: 10.21769/BioProtoc.2099 Views: 12630
Reviewed by: Ivan ZanoniShanie Saghafian-HedengrenAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Participant-Derived Xenograft Mouse Model to Decode Autologous Mechanisms of HIV Control and Evaluate Immunotherapies
Emma Falling Iversen [...] R. Brad Jones
Apr 5, 2025 2530 Views

PBMC-Humanized Mouse Model for Multiple Sclerosis: Studying Immune Changes and CNS Involvement
Anastasia Dagkonaki [...] Lesley Probert
May 20, 2025 3991 Views

Intraepidermal Nerve Fiber Quantification of the Mouse Hind Paw Footpads: A Detailed and Simplified Protocol
Anastasia Yerushkin [...] Amir Dori
Dec 5, 2025 1247 Views
Abstract
CD4+ T cell responses have been shown to be protective in many respiratory virus infections. In the respiratory tract, CD4+ T cells include cells in the airway and parenchyma and cells adhering to the pulmonary vasculature. Here we discuss in detail the methods that are useful for characterizing CD4+ T cells in different anatomic locations in mouse lungs.
Keywords: Memory CD4+ T cellBackground
To distinguish memory T cells in the circulation and tissues, a method for intravascular staining of T cells has been developed (Anderson et al., 2012). This method has been widely used to define memory T cells in many organs and tissues, including lungs, spleens and intestines. However, memory T cells in the respiratory tract are located in three unique anatomic locations, i.e., airway, parenchyma and pulmonary vasculature. Intravascular staining cannot distinguish cells in the airway and parenchyma, since they are both isolated from the circulation and intravascularly-administered antibodies will not stain these two populations. We designed a simultaneous intranasal/intravascular antibody labeling assay that can label and distinguish cells in all three locations using minimal amount of antibodies.
Materials and Reagents
- 1.5 ml microtubes (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 69715 )
- 1 ml insulin syringe fitted with 28 G x ½ needle (BD, catalog number: 329420 )
- Precision glide needles (20 G x 1) (BD, catalog number: 305175 )
- 5 ml polystyrene round bottom tubes (Corning, Falcon®, catalog number: 352054 )
- Precision glide needles (25 G x 5/8) (BD, catalog number: 305122 )
- 200 μl tips (Thermo Fisher Scientific, InvitrogenTM, catalog number: AM12655 )
- 15 ml screw cap conical tubes (SARSTEDT, catalog number: 62.554.002 )
- 10 ml syringes (BD, catalog number: 309604 )
- 12 well cell culture plates (Corning, Costar®, catalog number: 3513 )
- 3 ml syringes (BD, catalog number: 309657 )
- 50 ml screw cap conical tubes (SARSTEDT, catalog number: 62.547.004 )
- Cell strainers (70 µm nylon) (Corning, Falcon®, catalog number: 352350 )
- 60 x 15 mm tissue culture dish (Corning, Costar®, catalog number: 353802 )
- 10 ml stripettes (Corning, Costar®, catalog number: 3548 )
- Gauze sponges (4 x 4 inch) (Pro Advantage by NDC, catalog number: P157118 )
- 0.2 μm filter (EMD Millipore, catalog numbers: SCGPU11RE and SLGP033RS )
- 2.5 ml graduate transfer pipettes (RPI, catalog number: 147501-1S )
- Absorbent pads (COVIDIEN, catalog number: 949 )
- Mouse
- CD45-brilliant violet 510 (BV510) (Clone: 30-F11) (BioLegend, catalog number: 103138 )
- 1x Dulbecco’s phosphate buffered saline (DPBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 14190-144 )
- CD90.2-APC-eFluor 780 (Clone: 53-2.1) (Affymetrix, eBioscience, catalog number: 47-0902 )
- Isoflurane (USP inhalation vapour, liquid) (Drugs, catalog number: 57319-559-06 )
- Ethanol (Sigma-Aldrich, catalog number: 459836 )
- Trypan blue solution (Thermo Fisher Scientific, GibcoTM, catalog number: 15250061 )
- CD16/32-PerCP/Cy5.5 (Clone: 93) (BioLegend, catalog number: 101324 )
- CD4-FITC (Clone: RM4-5) (BioLegend, catalog number: 100510 )
- 2,2,2-tribromoethanol (Sigma-Aldrich, catalog number: T48402 )
- 2-methyl-2-butanol (Sigma-Aldrich, catalog number: 152463 )
- Nano-pure water (Thermo Fisher Scientific, InvitrogenTM, catalog number: 10977015 )
- RPMI medium 1640 (Thermo Fisher Scientific, GibcoTM, catalog number: 11875093 )
- HEPES (1 M) (Thermo Fisher Scientific, GibcoTM, catalog number: 15630080 )
- L-glutamine (200 mM) 100x (Thermo Fisher Scientific, GibcoTM, catalog number: 25030081 )
- Fetal bovine serum (FBS) (Atlanta Biologicals, catalog number: S11150 )
- MEM non-essential amino acids solution 100x (Thermo Fisher Scientific, GibcoTM, catalog number: 11140050 )
- Sodium pyruvate 100x (Thermo Fisher Scientific, GibcoTM, catalog number: 11360070 )
- Penicillin/streptomycin 100x (Thermo Fisher Scientific, GibcoTM, catalog number: 15140122 )
- 2-mercaptoethanol (Sigma-Aldrich, catalog number: M6250 )
- Collagenase D (Roche Diagnostics, catalog number: 11088882001 )
- DNase I (Roche Diagnostics, catalog number: 10104159001 )
- Hank’s balanced salt solution (HBSS) (Thermo Fisher Scientific, GibcoTM, catalog number: 14025 )
- Sodium azide, NaN3 (AMRESCO, catalog number: 0639 )
- Potassium bicarbonate, KHCO3 (Sigma-Aldrich, catalog number: 237205 )
- Ammonium chloride, NH4Cl (Sigma-Aldrich, catalog number: A9434 )
- EDTA-Na2 (Sigma-Aldrich, catalog number: E9884 )
- BD Cytofix fixation solution (BD, catalog number: 554655 )
- Avertin (see Recipes)
- Complete RPMI 1640 medium (see Recipes)
- Digestion buffer (see Recipes)
- FACS buffer (see Recipes)
- ACK lysis buffer (see Recipes)
Equipment
- Desiccator (SP Scienceware - Bel-Art Products - H-B Instrument, model: Space Saver Vacuum Desiccators )
- Fume hood (LABSCAPE)
- Pipetman P10 (Eppendorf, model: Research plus )
- Pipetman P200 (Eppendorf, model: Research plus )
- Pipetman P1000 (Eppendorf, model: Research plus )
- Pipet aid (Eppendorf, model: Eppendorf Easypet 3 )
- Heat lamp (Whitehead Industrial, catalog number: 30715 )
- Mouse restrainer (Braintree Scientific, catalog number: TV-150 STD )
- Spray bottle with 70% ethanol (SKS Science Products, catalog number: 0185-11 )
- Surgical scissors (Sklar Surgical Instrument, catalog number: 47-1246 )
- Tweezers (Sklar Surgical Instrument, catalog number: 66-7644 )
- Polystyrene foam (Regular polystyrene box top)
- Rocker (Labnet, catalog number: S0500 )
- Refrigerated tabletop centrifuge (Beckman Coulter, model: Allegra 6R )
- Hemocytometer (Thermo Fisher Scientific, catalog number: 99503 )
- Flow cytometer (BD, model: FACSVerse)
Software
- Flowjo software (Version 10.0.7)
Procedure
- Simultaneous intranasal/intravascular antibody labeling (see Videos 1 and 2)
- Dilute 0.25 μg CD45-BV510 antibody in 100 μl DPBS in a 1.5 ml microtube (for intranasal labelling). Dilute 0.5 μg CD90.2-APC-eFuor 780 antibody in 300 μl DPBS in another 1.5 ml microtube and load the antibody into a 1 ml insulin syringe with a 28 G x ½ needle (for intravenous labelling). Place the microtube and the syringe on ice until use. (see Note 1)
- Put a plastic desiccator in a fume hood. Place some gauze in the bottom chamber followed by 5 ml of isoflurane. (see Note 2)
- Place a mouse on the grate inside the desiccator (see Note 3).
- Load a P200 micropipette with 100 µl of CD45-BV510 antibody.
- Immediately after the rate of respiration slows, pick up the mouse, hold it vertically and slowly deliver 100 µl of CD45-BV510 antibody onto the mouse nostrils. Hold the mouse upright so that the liquid on the nostrils is completely aspirated into the lungs.
- Place the mouse back in the cage. After 2 min, turn on the heat lamp over the cage and warm up the mouse for 1 min.
- Remove the mouse from the cage, put it in a mouse restrainer and inject 0.5 μg CD90.2-APC-eFuor 780 antibody in 300 μl DPBS through the tail vein with the 1 ml insulin syringe prepared in point 1.
- Release the mouse from the restrainer and immediately anesthetize the mouse by intraperitoneal injection of 300 µl of avertin solution. Secure the mouse in the palm of the hand and hold it horizontally, then insert the needle at a shallow angle tangential to the mouse and slowly dispense avertin solution into the peritoneal cavity.Video 1. Intranasal administration of antibodiesVideo 2. Intravenous administration of antibodies
- Dilute 0.25 μg CD45-BV510 antibody in 100 μl DPBS in a 1.5 ml microtube (for intranasal labelling). Dilute 0.5 μg CD90.2-APC-eFuor 780 antibody in 300 μl DPBS in another 1.5 ml microtube and load the antibody into a 1 ml insulin syringe with a 28 G x ½ needle (for intravenous labelling). Place the microtube and the syringe on ice until use. (see Note 1)
- Harvest cells from airway (see Video 3) Video 3. Harvest of bronchoalveolar fluid, to obtain airway resident cells
- Prepare a 1 ml syringe with 20 G x 1 needle. Carefully pass about 1.25 inches 20 G tubing onto the needle. Sharpen the end of the tubing with scissors. Load the syringe with 1 ml complete RPMI 1640 medium and put it on ice until use (Figure 1).
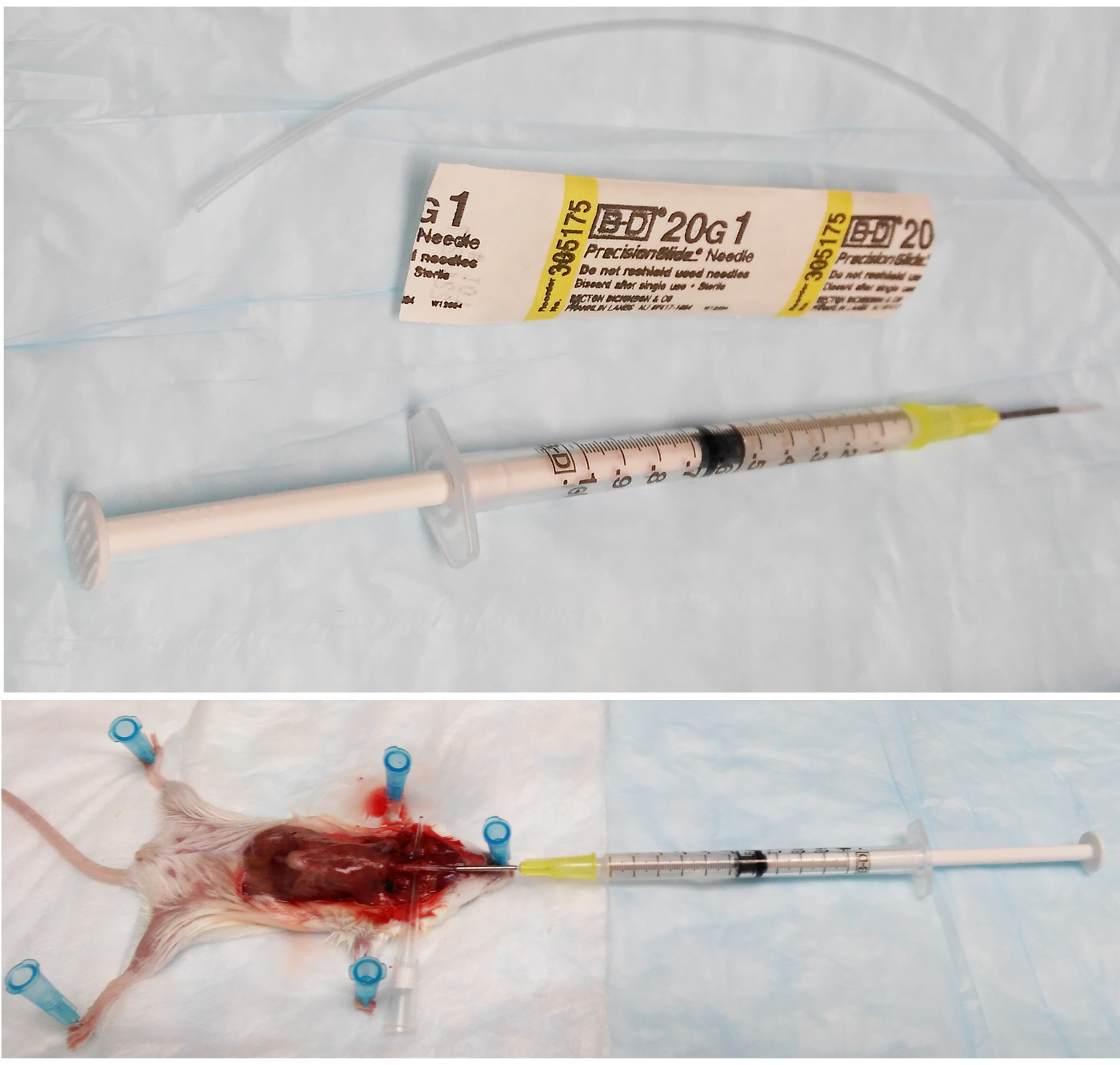
Figure 1. Representative pictures of syringe preparation and surgery for BALF lavage - When the mouse is fully anesthetized, typically within 2 min, immobilize it on a piece of polystyrene foam with absorbent pad on top by pinning each limb with a 25 G x 5/8 needle.
- Use a spray bottle filled with 70% ethanol to wet the fur of the mouse.
- Cut open the skin covering the abdominal and thoracic cavities through to the lower jaw. Use scissors to remove the tissue from the neck and expose the trachea.
- Use tweezers to separate the trachea from the underlying tissue and place a 200 μl tip under the trachea.
- Use a razor to cut through ½ of the trachea. Insert the 1 ml syringe with 20 G x 1 needle into the incision. Lavage for 4 times with complete RPMI 1640 medium. Collect the bronchoalveolar lavage fluid (BALF) in a 15 ml conical tube on ice. Typically 3-3.5 ml of BALF is recovered (Figure 1).
- Spin down cells for 5 min at 400 x g at 4 °C in a bucket tabletop centrifuge and resuspend the cells in 1 ml of ice cold FACS buffer (see Note 5).
- Prepare a 1 ml syringe with 20 G x 1 needle. Carefully pass about 1.25 inches 20 G tubing onto the needle. Sharpen the end of the tubing with scissors. Load the syringe with 1 ml complete RPMI 1640 medium and put it on ice until use (Figure 1).
- Sacrifice the mouse and harvest cells from lung (see Video 4)Video 4. Perfusing and harvesting lungs for cellular analysis
- After collection of BALF, cutting open the abdominal cavity to expose the underside of the diaphragm. Cut through the diaphragm with scissors and then remove the ribcage to fully expose the heart and lung.
- Fill a 10 ml syringe with ice cold sterile DPBS and attach a 25 G x 5/8 needle. Insert the needle into the left ventricle of the heart and smoothly dispense 5 ml of DPBS into the heart. In the meantime, use tweezers to break the right atria to allow blood to drain from circulation. Remove the needle from the left ventricle and insert it into the right ventricle to directly perfuse the lung with the remaining 5 ml of DPBS. (see Note 6)
- Cut the heart away from the lung and then remove the lung from the thoracic cavity after cutting the trachea and any remaining connective tissue.
- Place the lung into the well of a 12 well tissue culture plate filled with 2.5 ml of DPBS on ice.
- Rinse the lung with DPBS and transfer it into another well without DPBS. Mince the lungs into very fine pieces using scissors.
- Transfer minced lung with a 2.5 ml transfer pipette to 5 ml of digestion buffer in a 15 ml conical tube.
- Place tubes on a rocker and gently rotate at room temperature for 30 min in the dark.
- Place a 70 µm cell strainer into a 60 x 15 mm tissue culture dish.
- Transfer lung tissue in digestion buffer to the cell strainer using a 2.5 ml transfer pipette. Gently press and dissociate tissue through strainer with the flat end of a 3 ml syringe plunger. Process tissues until there is only connective tissue remaining on the strainer and rinse the strainer with complete RPMI 1640 medium. Transfer the resulting suspension to a 50 ml conical tube.
- Spin down lung cells for 5 min at 400 x g at 4 °C in a bucket tabletop centrifuge.
- Pour off supernatant and resuspend the cells in 3 ml of ACK buffer for 1 min to lyse the remaining red blood cells. Neutralize the ACK buffer with 30 ml of ice cold DPBS.
- Spin down the cells for 5 min at 400 x g at 4 °C and resuspend the cells in 5 ml of ice cold FACS buffer.
- After collection of BALF, cutting open the abdominal cavity to expose the underside of the diaphragm. Cut through the diaphragm with scissors and then remove the ribcage to fully expose the heart and lung.
- Cell staining
- Count the cells from BALF and lung using a hemocytometer in the presence of trypan blue.
- Spin down lung cells for 5 min at 400 x g at 4 °C and resuspend the cells in FACS buffer at 1 million cells per 50 µl.
- Spin down cells in the BALF for 5 min at 400 x g at 4 °C. Since much fewer cells are recovered from airway, resuspend 1-5 x 105 cells per 50 μl FACS buffer as needed.
- Dilute 0.25 μg CD4-FITC and 0.1 μg CD16/32 antibodies in 50 μl FACS buffer (see Note 4).
- Gently mix 50 μl cells and 50 μl antibodies together in a FACS tube.
- Incubate the cells in the dark for 15 min at 4 °C.
- Wash the cells once with 2 ml of FACS buffer at 400 x g for 5 min at 4 °C.
- Remove the supernatant and resuspend the cells in 100 µl FACS buffer.
- Pass the cells through a 70 µm cell strainer into new FACS tubes using a pipetman. Acquire FACS data using a flow cytometer and analyze data using Flowjo software (Figure 2). (see Note 7)
- Flow cytometry gating strategy
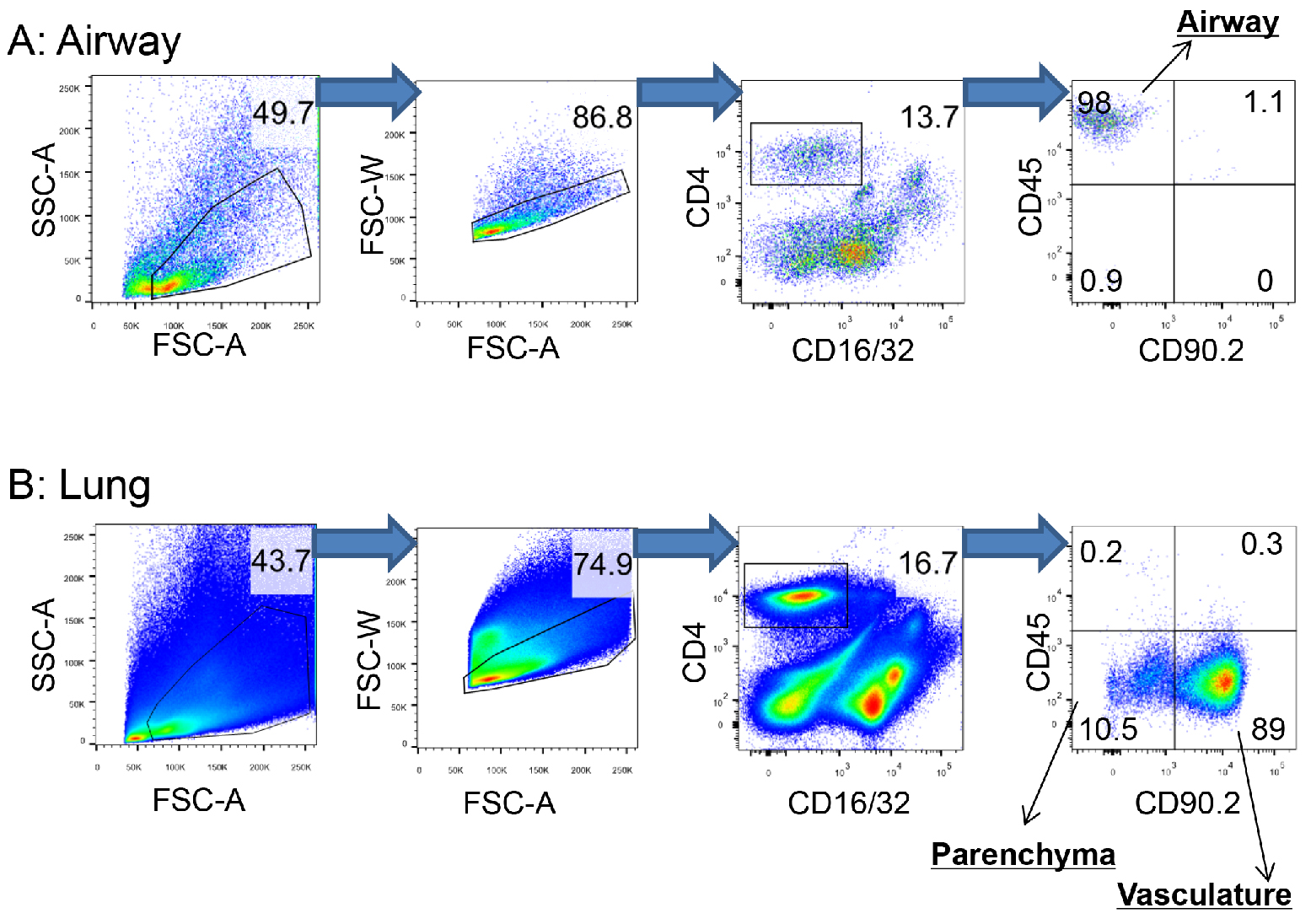
Figure 2. Gating strategy to distinguish CD4+ T cells in the airway, parenchyma and pulmonary vasculature. To localize memory CD4+ T cells in the respiratory tract, 0.25 μg of fluorochrome-conjugated CD45 and 0.5 μg of fluorochrome-conjugated CD90.2 antibody were injected into a SARS-CoV nucleocapsid protein-experienced mouse by i.n. and i.v. routes, respectively. Cells in the airway and lung were then harvested, stained and collected as described in Experimental Procedures and analysis using Flowjo software. A. CD4+ T cells in the airway: CD4+CD45+CD90.2-. B. CD4+ T cells in the parenchyma: CD4+CD45-CD90.2-. CD4+ T cells in the vasculature: CD4+CD45-CD90.2+. Data are representative of 10 independent experiments. (Zhao et al., 2016)
- Count the cells from BALF and lung using a hemocytometer in the presence of trypan blue.
Notes
- CD45 and CD90 are expressed by all CD4+ T cells. Both CD45 and CD90 antibodies can be used to stain CD4+ T cells in the lung and circulation. Phycoerythrin (PE), APC, BV (or their derivatives)-conjugated antibodies are preferentially chosen since these molecules have large molecular weights that will minimize airway/vascular leakage during in vivo labeling.
- Make sure to tape over the center hole in the plastic desiccator grate because small mice are sometimes able to squeeze through this hole and jump into the bottom, isoflurane-filled chamber.
- Here we used an antigen-experienced mouse that had been immunized with a construct expressing the SARS-CoV nucleocapsid protein (Zhao et al., 2016). This protocol can be applied to study memory and effector as well as naïve CD4+ T cells in mouse lungs.
- Cells can also be stained with other phenotypic (surface) antibodies or cultured for intracellular cytokine staining as previously described (Fett et al., 2014).
- For phenotypic staining, cells will be resuspended in FACS buffer. For intracellular staining, cell will be resuspended in complete RPMI 1640 medium.
- The exterior surface of the lung will turn white after a good perfusion. Generally 5 ml PBS is sufficient.
- This step is necessary to prevent clots formation in the flow cytometer. Other flow cytometers, such as LSR, Fortessa… can also be used to acquire cells.
Recipes
- Avertin (250 ml)
5 g 2,2,2-tribromoethanol
5 ml 2-methyl-2-butanol
225 ml hot nano-pure water (95 °C)
Stir for 2 h in a fume hood and adjust volume to 250 ml
Filter through a 0.2 μm filter, aliquot and store at -20 °C - Complete RPMI 1640 medium (500 ml)
417 ml RPMI 1640 medium
12.5 ml 1 M HEPES
5 ml 100x L-glutamine
50 ml FBS
5 ml 100x MEM non-essential amino acids
5 ml 100x sodium pyruvate
5 ml 100x penicillin/streptomycin
0.5 ml 2-mercaptoethanol (50 mM in nano-pure H2O, 0.2 μm filtered)
Store at 4 °C - Digestion buffer (100 ml)
100 mg collagenase D
10 mg DNase I
2 ml FBS
1 ml 100x glutamine
2.5 ml HEPES (1 M)
1 ml 100x penicillin/streptomycin
Adjust volume to 100 ml with HBSS
Aliquot and store at -20 °C - FACS buffer (500 ml)
1.7 ml sodium azide (from 30% stock made in nano-pure H2O)
15 ml FBS
483.3 ml DPBS
Store at 4 °C - ACK lysis buffer
1 g KHCO3
8.3 g NH4Cl
37.2 mg EDTA-Na2
Adjust volume to 1,000 ml with nano-pure H2O and filter through a 0.2 μm filter
Store at room temperature
Acknowledgments
This work was supported by the Thousand Talents Plan Award of China 2015 (J.Z.), the Municipal Healthcare Joint-Innovation Major Project of Guangzhou (201604020011) (J.Z.) and the N.I.H. (USA) (PO1 AI060699, S.P.). The protocol described herein was based on the following manuscript: Zhao et al. Immunity. 2016 Jun 21; 44(6):1379-91.
References
- Anderson, K. G., Sung, H., Skon, C. N., Lefrancois, L., Deisinger, A., Vezys, V. and Masopust, D. (2012). Cutting edge: intravascular staining redefines lung CD8 T cell responses. J Immunol 189(6): 2702-2706.
- Fett, C., Zhao, J. and Perlman, S. (2014). Measurement of CD8 and CD4 T cell responses in mouse lungs. Bio Protoc 4(6).
- Zhao, J., Zhao, J., Mangalam, A. K., Channappanavar, R., Fett, C., Meyerholz, D. K., Agnihothram, S., Baric, R. S., David, C. S. and Perlman, S. (2016). Airway memory CD4+ T cells mediate protective immunity against emerging respiratory coronaviruses. Immunity 44(6): 1379-1391.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Wang, Y., Sun, J., Channappanavar, R., Zhao, J., Perlman, S. and Zhao, J. (2017). Simultaneous Intranasal/Intravascular Antibody Labeling of CD4+ T Cells in Mouse Lungs. Bio-protocol 7(1): e2099. DOI: 10.21769/BioProtoc.2099.
Category
Immunology > Animal model > Mouse
Cell Biology > Cell staining > Protein
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









