- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Protocol for Increasing Carotenoid Levels in the Roots of Citrus Plants
Published: Vol 6, Iss 24, Dec 20, 2016 DOI: 10.21769/BioProtoc.2077 Views: 8559
Reviewed by: Scott A M McAdamLaia ArmengotAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Quantification of Ethylene Production in Leaf and Bud Tissue of the Subtropical Tree Crop Litchi (Litchi chinensis Sonn.) Using Gas Chromatography and Flame Ionization Detection
Regina B. Cronje and Arnoldus J. Jonker
Mar 20, 2023 1561 Views

Bi-directional Dual-flow-RootChip for Physiological Analysis of Plant Primary Roots Under Asymmetric Perfusion of Stress Treatments
Claudia Allan [...] Claudia-Nicole Meisrimler
Aug 5, 2023 1963 Views

Enzymatic Starch Quantification in Developing Flower Primordia of Sweet Cherry
Nestor Santolaria [...] Afif Hedhly
Apr 5, 2025 1906 Views
Abstract
Carotenoids in plants play several key functions such as acting as light-harvesters, antioxidants (Lado et al., 2016) or being precursors of strigolactones, abscisic acid, volatiles and other signaling compounds (Arbona et al., 2013). Although those functions are well-known in light-exposed tissues, information in belowground organs is limited because of reduced abundance of these pigments. In order to better understand the role of carotenoids in roots, we developed a methodology to increase the abundance of these pigments in underground tissues. We took advantage of the fact that citrus roots exposed to light develop pigmentation in order to increase the carotenoid content. Therefore, here we describe a simple method to increase carotenoids in citrus roots.
Keywords: Abscisic acidBackground
Carotenoid abundance in roots is quite limited and, therefore, understanding the role of these compounds becomes difficult. Exposure of roots to light is a simple, fast and useful tool to increase carotenoid levels in these tissues, especially when compared to other genomic approaches such as overexpressing some key genes of the carotenoid biosynthetic pathway (Cao et al., 2015).
Materials and Reagents
- Pirex® culture tubes 25 x 150 mm
- Disposable syringes (25 ml)
- 1.5 ml Eppendorf tubes
- Citrus seeds (e.g., from a commercial rootstock)
- Murashige and Skoog (MS) medium (4,302.09 mg L−1) (Duchefa Biochemie, catalog number: M0221 )
- Sucrose (30 g L−1) (any supplier)
- Sterile MiliQ-water
- Agar (European Bacteriological Agar) (Conda, Pronadisa, catalog number: 1800 )
- Tween® 20 (0.1% v/v) (Sigma-Aldrich, catalog number: P2287 )
- Myo-inositol (100 mg L−1) (Duchefa Biochemie, catalog number: I0609 )
- Pyridoxine-HCl (1.0 mg L−1) (Duchefa Biochemie, catalog number: P0612 )
- Thiamine-HCl (0.2 mg L−1) (Duchefa Biochemie, catalog number: T0614 )
- Nicotinic acid (0.5 mg L−1) (PANREAC QUÍMICA, catalog number: A0963 )
- Glycine (0.2 mg L−1) (PANREAC QUÍMICA, catalog number: 141340 )
- Mix A (see Recipes)
- Sodium hypochlorite solution (50.0%, v/v) (see Recipes)
- NaOH (0.1 N) (any supplier) (see Recipes)
Equipment
- Pirex® media solution bottles
- Autoclave (Raypa, model: Steam Sterilizer AES-75 )
- Laminar flow hood (Bioquell, ASTEC MICROFLOW, model: Microflow Laminar Flow Workstation M50546 )
- Hooked tweezers
- Growth chamber (Snijders Scientific, model: Economic Lux Climate Chamber )
- Long-handled scissors with curved tip
- pH meter (HACH LANGE SPAIN, model: pH meter Basic 20 )
Procedure
- Prepare growing medium
- Dissolve 4.3 g L-1 of MS, 1.0 ml of mix A (see Recipes) and 30 g L-1 of sucrose in distilled water.
- Adjust the pH of the solution to 5.7 ± 0.1 with NaOH (0.1 N).
- Distribute the solution in bottles for autoclaving.
- Autoclave the solution at 105 °C for 5 min.
- Dissolve 9.0 g L-1 of agar to solidify the medium.
- Even with the warm medium and by means of syringes, dispense 25 ml of the solution into the culture tubes and cover it.
- Autoclave the racks containing tubes for 15 min at 115 °C.
- Let it cool down and store at room temperature.
- Dissolve 4.3 g L-1 of MS, 1.0 ml of mix A (see Recipes) and 30 g L-1 of sucrose in distilled water.
- Plant material preparation
- Remove the coats of the seeds to increase and homogenize seed germination. Avoid damaging the seeds; especially pay attention not to injure the embryo (Figure 1). Disinfect the seeds by putting them into a sodium hypochlorite solution (50.0%, v/v) plus 0.1% (v/v) Tween® 20 for 10 min.
- Rinse the seeds three times with sterile distilled water in order to remove hypochlorite solution.
- Store the seeds in a sterile laminar flow hood preventing dehydration.

Figure 1. Example of seeds coat removal for increasing the rate and homogenization of germination
- Remove the coats of the seeds to increase and homogenize seed germination. Avoid damaging the seeds; especially pay attention not to injure the embryo (Figure 1). Disinfect the seeds by putting them into a sodium hypochlorite solution (50.0%, v/v) plus 0.1% (v/v) Tween® 20 for 10 min.
- Germination and growing conditions
- Working in a sterile laminar flow hood and by means of a sterile tweezer, place one seed per tube in the solidified growing medium and cover the tubes after sown (Figure 2A).
- Once seeds are sown place racks into a growing chamber for two weeks maintained at 25 °C and darkness to promote germination (Figure 2B).
- After two weeks, transfer the etiolated seedlings (of about 5 cm in large at that time) to a lighted growth chamber. Set photoperiod conditions of 16:8 h (light:dark), temperature of 25 °C and a minimum radiation of 150 µmol m-2 s-1 photosynthetically active radiation (PAR) (Figure 2C).
- After three weeks under light conditions when plants have grown and turned to green coloration, shoot needs to be removed. In the sterile laminar flow hood plants are detached from shoot by means of long-handled scissors (Figure 3). Do this under sterile conditions in the laminar flow hood. Shoots are removed by cutting about 5 mm below of the root/shoot junction, keeping the remaining root inside the medium to avoid future shoot regrowth (Figure 2D).
- Tubes containing root tissues are maintained for at least two days prior to experiments are performed to exclude any side-effect of wounding (Figure 4).
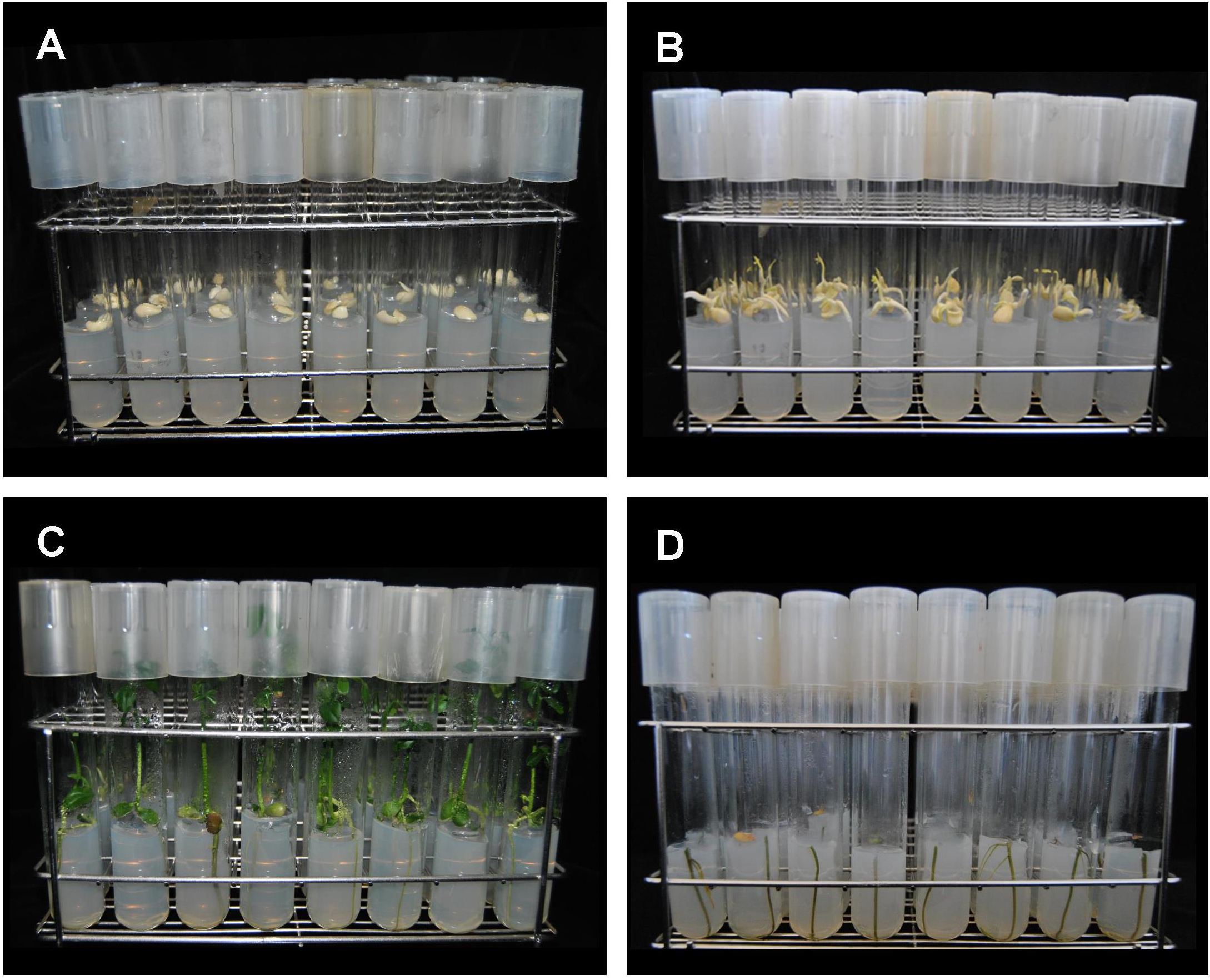
Figure 2. Example of seedling development under in vitro condition. A. Sown seeds. B. Etiolated seedlings growing under dark conditions. C. Seeds turned green after 3 weeks under illumination. D. Detail of green roots after removal of shoots just under the shoot/root junction.
Figure 3. Detail of long-handled scissors used for detaching roots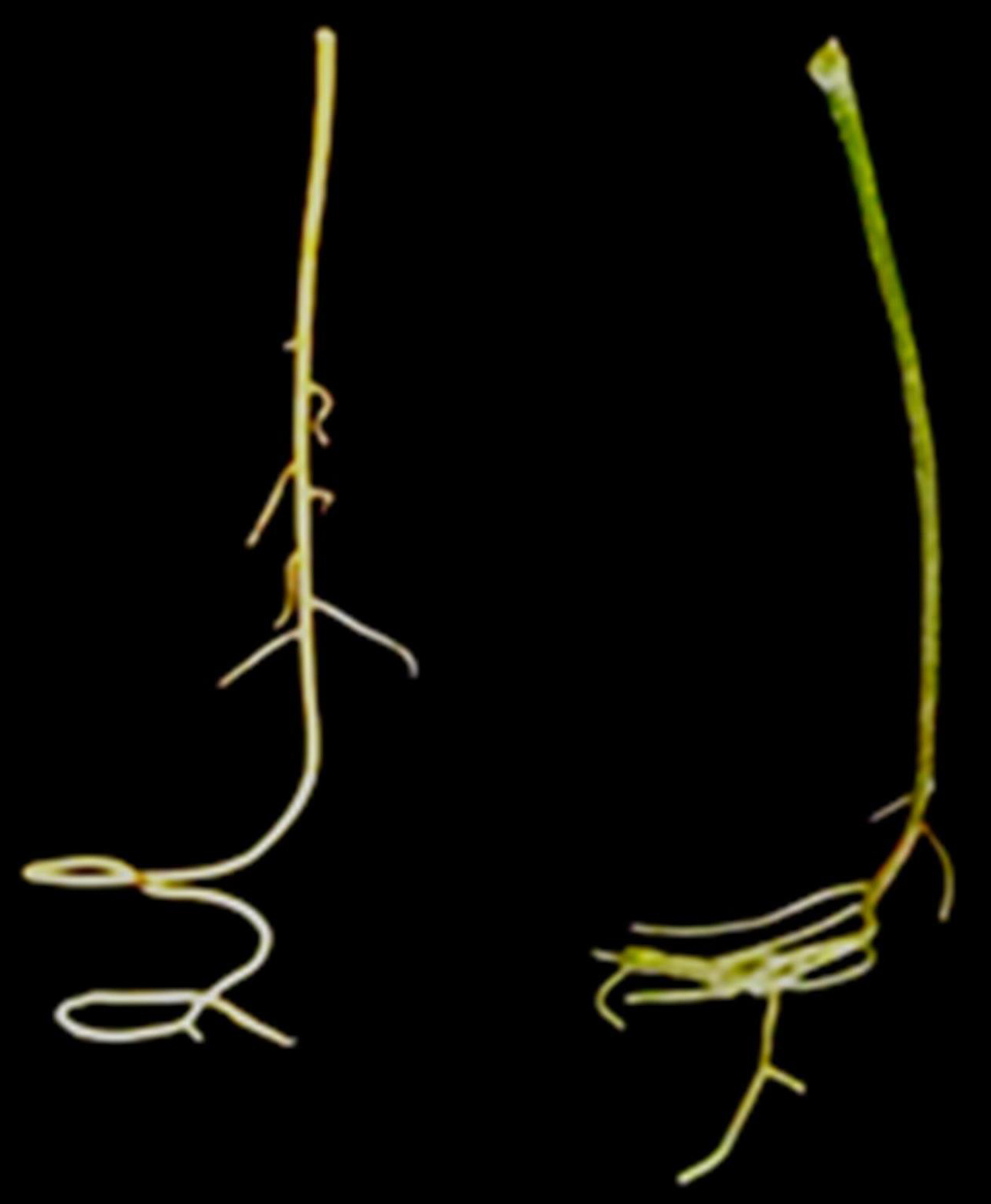
Figure 4. Detail of the coloration of detached root in response to dark (right) and light (left) exposure
- Working in a sterile laminar flow hood and by means of a sterile tweezer, place one seed per tube in the solidified growing medium and cover the tubes after sown (Figure 2A).
Data analysis
The aim of this protocol is to increase the levels of carotenoids in roots. To evaluate the raise of these pigments two alternative methodologies could be used: on one hand, spectrophotometric analysis could be performed by many different protocols, evaluating in this case the total carotenoid content (i.e., Wellburn, 1994). Alternatively, detailed carotenoid composition could be achieved by liquid chromatography coupled to a diode array detector (HPLC-DAD) as detailed in Manzi et al., (2016). Carotenoid quantification should be performed accordingly to the method used.
Notes
Rates of growth may differ among citrus genotypes. To avoid unwanted effects on metabolism, avoid excessive growing of roots which may lead to an early senescence. To this, you might adjust the growing time of the plants accordingly (i.e., reducing the 3 week period under light conditions).
In order to obtain high levels of sample material a good option is increasing the number of individual roots rather than extending the root growing period. Consider that 20 roots provide approximately 1.5-2.0 g of fresh tissue.
Recipes
- Mix A
Dilute a mix of myo-inositol (100 mg L-1), pyridoxine-HCl (1.0 mg L-1), thiamine-HCl (0.2 mg L-1), nicotinic acid (0.5 mg L-1) and glycine (0.2 mg L-1) in distilled water
Aliquot in 1.5 ml Eppendorf tubes
Aliquots must be stored at -20 °C
Note: One of this Eppendorf (1.5 ml) is added for each 1.5 L of growing medium - Sodium hypochlorite solution (50.0%, v/v)
Mix equal parts of pure sodium hypochlorite (100%) and distilled MiliQ water
Tween® 20 wetting agent at 0.1% (v/v) is added to the final solution
This solution can be either stored or prepared in the moment
Note: The volume of solution to prepare depends on the number of seeds to disinfect. To avoid any harmful effect of the hypochlorite, seeds must not be exposed to the disinfectant solution for more than 10 min - NaOH (0.1 N)
Dissolve 40 g of NaOH in 1.0 L of distilled water to make a 1.0 N solution
This solution must be stored at 4 °C
At the time of preparation of 0.1 N solution, take 1.0 ml of 1.0 N NaOH solution and complete to 10 ml by adding 9.0 ml of distilled water
Note: Add drop by drop of 0.1 N NaOH to adjust the pH of the germination medium. Usually, only few drops are sufficient to achieve a pH = 5.7 ± 0.1 of 1.0 L of medium
Acknowledgments
This work was supported by the Ministerio de Economia (MINECO) and Universitat Jaume I through grants No. AGL2013- 42038-R and P1IB2013-23, respectively. MM was recipient of a ‘Santiago Grisolia’ fellowship from Generalitat Valenciana (Spain). This protocol is based on the methodology used in the manuscript Manzi et al. (2016).
References
- Arbona, V., Manzi, M., Ollas, C. and Gomez-Cadenas, A. (2013). Metabolomics as a tool to investigate abiotic stress tolerance in plants. Int J Mol Sci 14(3): 4885-4911.
- Cao, H., Wang, J., Dong, X., Han, Y., Ma, Q., Ding, Y., Zhao, F., Zhang, J., Chen, H., Xu, Q., Xu, J. and Deng, X. (2015). Carotenoid accumulation affects redox status, starch metabolism, and flavonoid/anthocyanin accumulation in citrus. BMC Plant Biol 15: 27.
- Lado, J., Rodrigo, M. J., López-Climent, M., Gómez-Cadenas, A. and Zacarías, L. (2016). Implication of the antioxidant system in chilling injury tolerance in the red peel of grapefruit. Postharvest Biol Tec 111: 214-223.
- Manzi, M., Lado, J., Rodrigo, M. J., Arbona, V. and Gomez-Cadenas, A. (2016). ABA accumulation in water-stressed Citrus roots does not rely on carotenoid content in this organ. Plant Sci 252: 151-161.
- Wellburn, A. R. (1994). The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144: 307–313.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Manzi, M., Pitarch-Bielsa, M., Arbona, V. and Gómez-Cadenas, A. (2016). Protocol for Increasing Carotenoid Levels in the Roots of Citrus Plants. Bio-protocol 6(24): e2077. DOI: 10.21769/BioProtoc.2077.
Category
Plant Science > Plant physiology > Plant growth
Biochemistry > Other compound > Carotenoid
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









