- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolation of Primary Breast Cancer Cells from HER2 Transgenic Mice
Published: Vol 6, Iss 19, Oct 5, 2016 DOI: 10.21769/BioProtoc.1956 Views: 14746
Reviewed by: HongLok LungShravani MukherjeeAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Isolation of Stem Cells, Endothelial Cells and Pericytes from Human Infantile Hemangioma
Lan Huang and Joyce Bischoff
Jan 20, 2020 4962 Views

Low-viscosity Matrix Suspension Culture for Human Colorectal Epithelial Organoids and Tumoroids
Tao Tan [...] Oliver M. Sieber
Apr 20, 2022 4057 Views

Thrombopoietin-independent Megakaryocyte Differentiation of Hematopoietic Progenitor Cells from Patients with Myeloproliferative Neoplasms
Chloe A. L. Thompson-Peach [...] Daniel Thomas
Jan 20, 2023 2363 Views
Abstract
HER2 is a tyrosine kinase receptor, which is overexpressed in about 30% of breast cancer patients. Its overexpression leads to mammary tumorigenesis and increased invasion and metastasis (Slamon et al., 1987). HER2 transgenic mouse (FVB/N-MMTVneu mouse) is a well-established model of mammary tumor in human (Fantozzi and Christofori, 2006). Although in vivo models are excellent for assessing the influence of various factors, especially microenvironment, on development of breast cancer, a convenient and less costly way to study the underlying molecular events is utilizing cells derived from the model under evaluation. In order to explore the molecular mechanism by which HOXB7 inhibits initiation, but promotes metastasis of breast tumors, we generated mouse breast cancer cell line from HER2 transgenic mouse (Liu et al., 2015). This protocol may be useful for the generation of breast cancer cell line from mice with other genetic backgrounds.
Keywords: HER2 Transgenic miceMaterials and Reagents
- Cell strainer, sterile (100 μm) (Corning, Falcon®, catalog number: 352360 )
- 10 cm standard tissue culture dish (Corning, catalog number: 430293 )
- 50 ml conical tubes, sterile (Corning, Falcon®, catalog number: 352070 )
- Disposable scalpels, forceps and scissors (VWR International)
- HER2 transgenic mice (THE JACKSON LABORATORY, catalog number: 002376 )
- 0.25% trypsin-EDTA (1x) (Thermo Fisher Scientific, GibcoTM, catalog number: 25200-056 )
- Phosphate-buffered saline (PBS), sterile (self-preparation)
- HEPES (Sigma-Aldrich, catalog number: H3375 )
- DMEM/F12-with L-glutamine (Thermo Fisher Scientific, GibcoTM, catalog number: 11875-093 )
- Bovine serum albumin solution (BSA) (Sigma-Aldrich, catalog number: A9576 )
- Hydrocortisone (Sigma-Aldrich, catalog number: H0888 )
- Type IV collagenase (Sigma-Aldrich, catalog number: C5138 )
- Hyaluronidase (Sigma-Aldrich, catalog number: H3884 )
- Pen-Strep (GE Healthcare, HyCloneTM, catalog number: SV30010 )
- Ammonium chloride (NH4Cl) (Sigma-Aldrich, catalog number: A9434 )
- Tris (Sigma-Aldrich, catalog number: 93362 )
- Fetal bovine serum (FBS), heat inactivated (GE Healthcare, HyCloneTM, catalog number: SH30071.03 )
- Insulin (with transferrin/selenium) (Thermo Fisher Scientific, GibcoTM, catalog number: 51500-056 )
- Digestion buffer (see Recipes)
- Tris-buffered ammonium chloride (TAC buffer) (see Recipes)
- Complete media (see Recipes)
Equipment
- Shaking incubator (Thermo Fisher Scientific, Thermo Scientific, model: SHKA5000 )
- 37 °C, 5% CO2 cell culture incubator (VWR, symphonyTM, model: 5.3A )
- Pipette
- Refrigerated centrifuge (Eppendorf, model: 5418R )
- Water bath
- Hemocytometer
- Inverted microscope (Olympus Corporation, model: CKX41 )
- Tissue culture hood equipped with UV light source (Labconco, model: Purifier Logic+ Class II , Type A2 Biosafety Cabinet)
Procedure
- Processing solid tumor tissue into single cells
- Take out tumor from mouse mammary gland using forceps and scissors (Figure 1), and place it in a 10 cm Petri dish, pouring sterile DMEM/F12 medium on the tissue to keep it moist.

Figure 1. Spontaneous breast tumor in MMTV-HER2 mice
- Cut the tumor into pieces using a scalpel, removing necrotic tissue if present. Necrotic tissue is normally darker and softer compared to the surrounding tumor.
- Mince the tissue using disposable scalpels until finely chopped (Figure 2A and 2B), and transfer the minced tissue to a 50 ml conical vial.
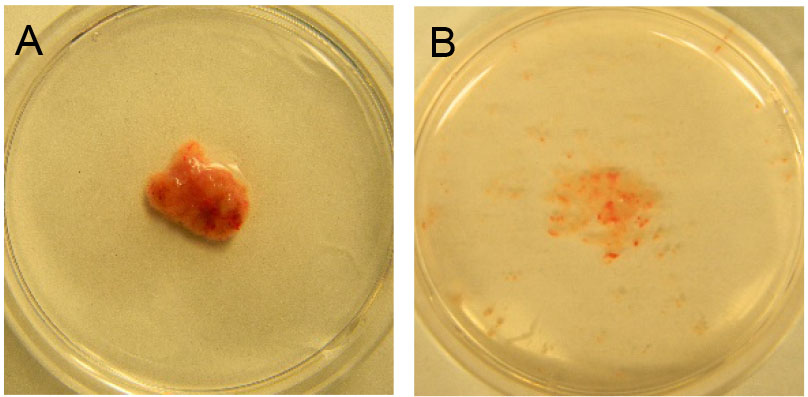
Figure 2. Tumor tissue from spontaneous tumor. A. Before mincing; B. After mincing. - Add 10 ml digestion buffer (about 0.5 cm3 in 10 ml digestion buffer).
- Close the tube with a cap and wrap the cap with Parafilm. Place the tube in a 37 °C shaker at low to moderate speed (e.g., 100-200 rpm) for 1-2 h.
- Pipette up and down the digested tissue and centrifuge at 530 x g at room temperature for 5 min to pellet the cells.
- Aspirate the supernatant, which contains fat. If the pellet contains red blood cells (observed as a red layer on top of the pellet), resuspend the pellet in 5-10 ml of TAC buffer and incubate 3-10 min in a 37 °C water bath. Centrifuge at 530 x g at room temperature for 5 min. Repeat this step until the red blood cells are no longer visible.
- Resuspend the pellet in 10 ml of DMEM/F12 medium (room temperature, without supplements). Filter the digested tissue using cell strainer.
Note: It is not necessary. Normally, I do it because I need to use it for FACS or sorting. - Centrifuge at 530 x g at room temperature for 1 min. Resuspend the pellet in 10 ml of DMEM/F12 medium (room temperature, without supplements).
- Perform a trypan blue exclusion test using a hemocytometer to determine the number of viable cells per ml.
- Seed 4-6 x 106 viable cells into 10 cm dish with complete media. Add 1x Pen-Strp.
- Take out tumor from mouse mammary gland using forceps and scissors (Figure 1), and place it in a 10 cm Petri dish, pouring sterile DMEM/F12 medium on the tissue to keep it moist.
- Generating tumor cell line
- Split cells once it is confluent. Normally the cells grow very fast in the first 3 passages.
- Purify tumor cells via removing fibroblast. Fibroblasts are often more sensitive to trypsin and can therefore be removed from the plate while the tumor cells adhere for a longer time. So treating the cells with trypsin (normally 2-3 min at 37 °C), and constantly monitor it under a microscope. Once the fibroblast (blue arrowhead) detached from the plate, while the tumor cells (red arrowhead) still adhere on the plate (Figure 3A and 3B), gently suck trypsin and wash with PBS. Then trypsinize and transfer the tumor cells to a new plate. After repeating this step for several passages, the majority of cells are tumor cells.
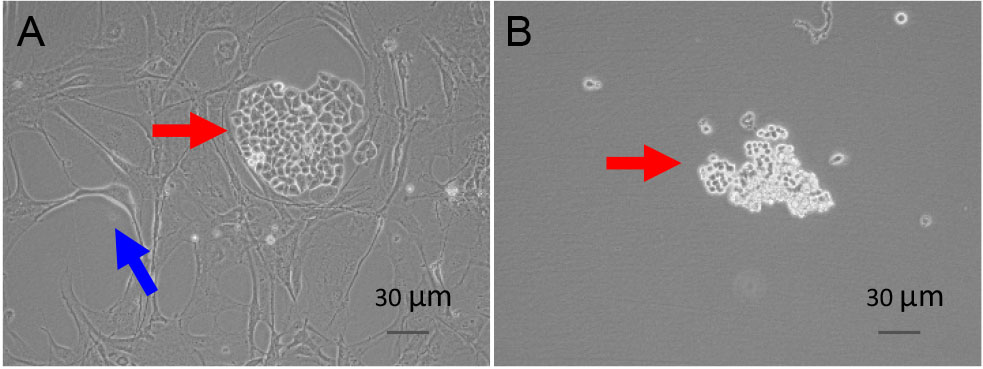
Figure 3. Purification of tumor cells. A. Before trypsinization; B. After trypsinization. Blue arrowhead: fibroblast; red arrowhead: tumor cells. - Passaging cells every 5-7 days after 3-4 passages. In the course of passage, the cells grow slowly or even no growth. Over 90% of cells die.
- After passaging 8-9 times, the cells are immortalized, and grow faster and faster. Figure 4 shows the morphology of tumor cells at different passage.
- The morphology of generated tumor cell line is shown in Figure 5. And the expression of HER2 is examined by IHC (Figure 6A) and Western (Figure 6B).
- The tumorigenicity of generated tumor cell line is tested by inoculating cells into the mammary gland fat pad of HER2 transgenic mice (Figure 7).
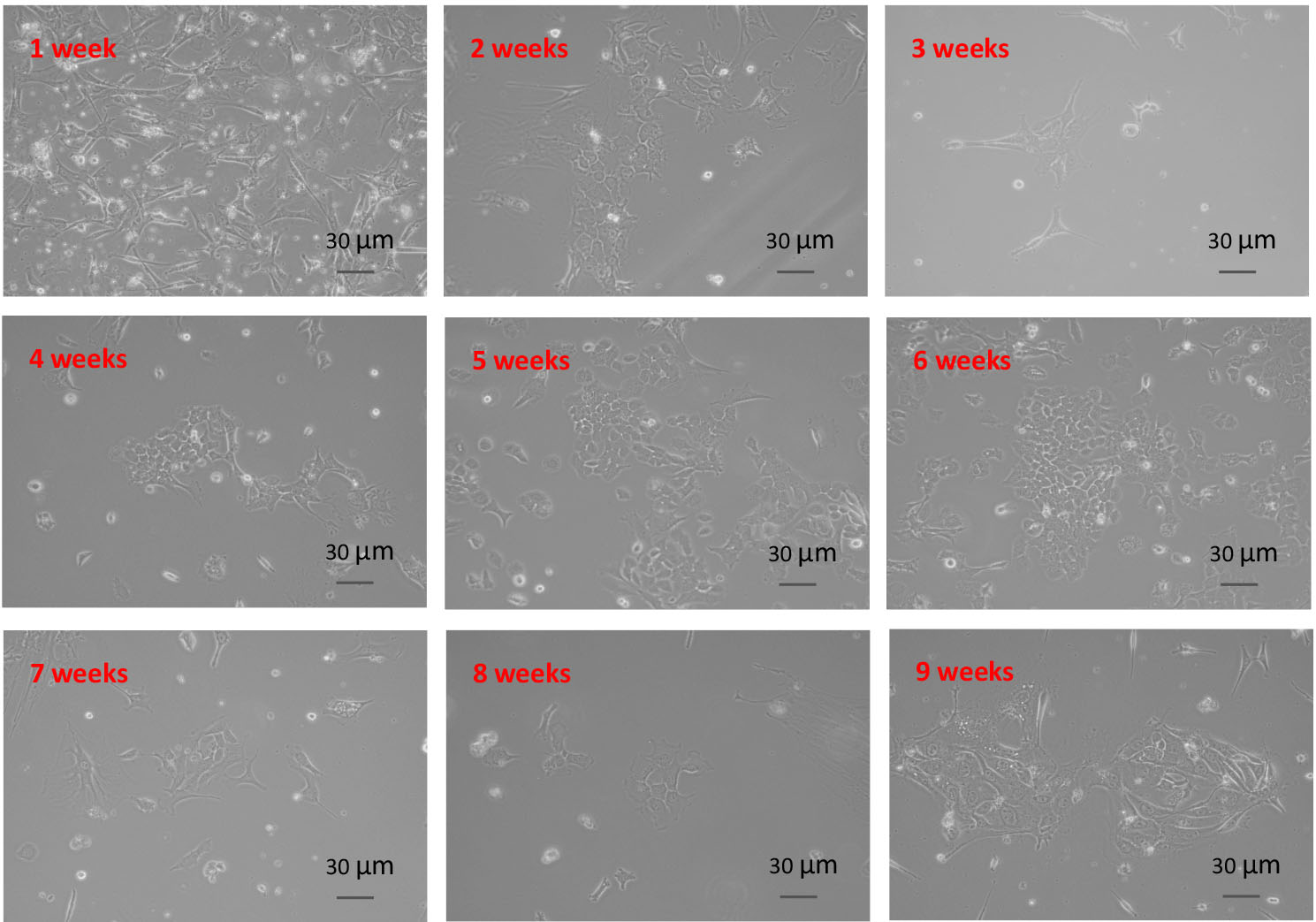
Figure 4. Morphology of tumor cells at different passages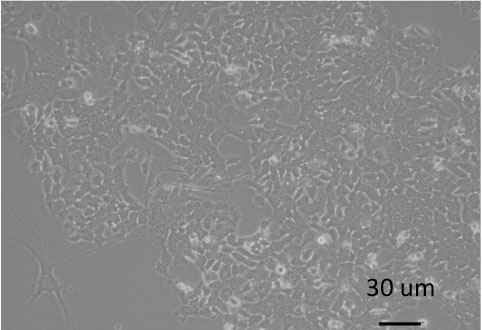
Figure 5. Representative tumor cell line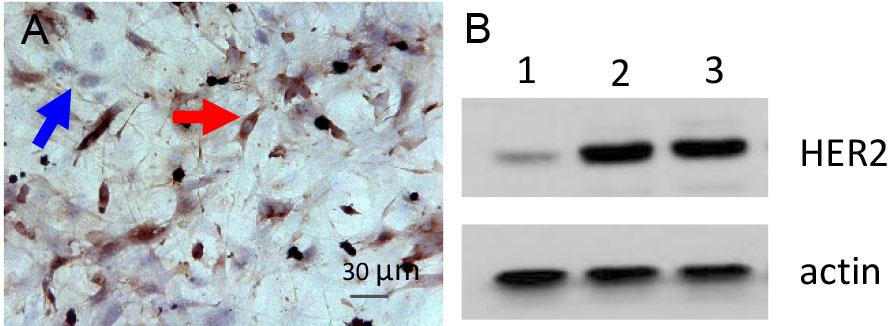
Figure 6. HER2 expression in tumor cells. A. IHC staining for HER2 in tumor cells (blue arrowhead: fibroblast; red arrowhead: tumor cells). B. HER2 expression in tumor cell line (1: MCF10A-Vector; 2: MCF10A-HER2; 3: Representative cell line).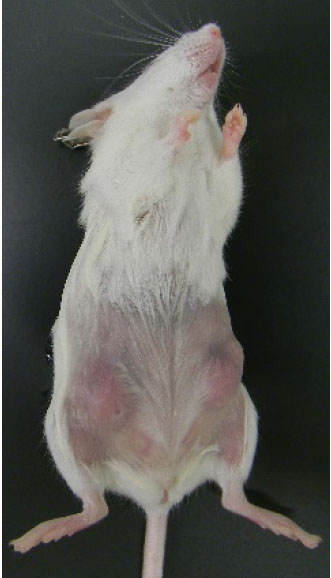
Figure 7. Tumorigenicity of established tumor cell line. Tumors formed in HER2 transgenic mouse after inoculating representative cell line in 104/site for 4 weeks.
- Split cells once it is confluent. Normally the cells grow very fast in the first 3 passages.
Notes
- Based on what I did, stable cell lines can be generated in about 30% of tumor tissues.
- The cells start to proliferate after passaging 8-9 times or more. I think it is a sign for immortalization. However, I do not know the underlying mechanism.
Recipes
- Digestion buffer
DMEM-F/12 supplemented with:
10 mM HEPES
2% BSA
0.5 μg/ml hydrocortisone
1 mg/ml IV collagenase
0.1 mg/ml hyaluronidase
1x Pen-Strp
Sterile filter and store at 4 °C. - Tris-buffered ammonium chloride (TAC buffer)
0.16 M NH4Cl
0.17 M Tris
Adjust pH to 7.65 with HCl
To make the working solution:
Mix 90 ml of 0.16 M NH4Cl and 10 ml of 0.17 M Tris
Adjust to pH 7.2 with HCl
Sterile filter and store at 4 °C. - Complete media
DMEM-F/12 supplemented with 10% FBS and 10 μg/ml insulin.
Acknowledgments
This work was supported by the American Cancer Society Research Award (RSG-10-067-01-TBE) to Hexin Chen.
References
- Fantozzi, A. and Christofori, G. (2006). Mouse models of breast cancer metastasis. Breast Cancer Res 8(4): 212.
- Liu, S., Jin, K., Hui, Y., Fu, J., Jie, C., Feng, S., Reisman, D., Wang, Q., Fan, D., Sukumar, S. and Chen, H. (2015). HOXB7 promotes malignant progression by activating the TGFβ signaling pathway. Cancer Res 75(4): 709-719.
- Slamon, D. J., Clark, G. M., Wong, S. G., Levin, W. J., Ullrich, A. and McGuire, W. L. (1987). Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235(4785): 177-182.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Liu, S. and Chen, H. (2016). Isolation of Primary Breast Cancer Cells from HER2 Transgenic Mice. Bio-protocol 6(19): e1956. DOI: 10.21769/BioProtoc.1956.
Category
Cancer Biology > Cancer stem cell > Cell biology assays > Cell isolation and culture
Cell Biology > Cell isolation and culture > Cell isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









