- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
In vivo Analysis of Neutrophil Infiltration during LPS-induced Peritonitis
Published: Vol 6, Iss 19, Oct 5, 2016 DOI: 10.21769/BioProtoc.1945 Views: 11076
Reviewed by: Ivan ZanoniAchille BroggiMarco Di Gioia

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Participant-Derived Xenograft Mouse Model to Decode Autologous Mechanisms of HIV Control and Evaluate Immunotherapies
Emma Falling Iversen [...] R. Brad Jones
Apr 5, 2025 2531 Views

PBMC-Humanized Mouse Model for Multiple Sclerosis: Studying Immune Changes and CNS Involvement
Anastasia Dagkonaki [...] Lesley Probert
May 20, 2025 3993 Views

Utilizing EdU to Track Leukocyte Recruitment to the Brain
Zoie K. Lipfert [...] David P. Sullivan
Dec 5, 2025 1564 Views
Abstract
Bacterial lipopolysaccharide (LPS) is present in the outer membrane of Gram-negative bacteria and functions as pathogen-associated molecular pattern (PAMP) (Whitfield and Trent, 2014). LPS therefore is a potent activator of inflammatory responses leading to cytokine release and neutrophils recruitment. The lipid A moiety of LPS activates the complex consisting of the LPS binding protein (LBP), CD14, MD-2 and Toll-like receptor 4 (TLR4) and the non-canonical inflammasome-linked caspases-4, 5 and 11, which in turn activate the canonical NLRP3 inflammasome (Shi et al., 2014; Hagar et al., 2013; Kayagaki et al., 2013; Hoshino et al., 1999; Poltorak, 1998; Nagai et al., 2002; Park et al., 2009; Ratsimandresy et al., 2013). In particular, the cytokine interleukin (IL)-1β produced in response to inflammasome activation has a crucial role in neutrophil recruitment through promoting neutrophil adhesion and migration (McDonald et al., 2010).This protocol allows studying of inflammatory response induced by LPS that affect neutrophil infiltration by tracking myeloperoxidase (MPO) activity in vivo (de Almeida et al., 2015).
Keywords: InflammationMaterials and Reagents
- Insulin syringes (Thermo Fisher Scientific, catalog number: 14-841-31 )
- 0.22 μm filters
- C57BL/6 mice, typically of 8-12 weeks old mice (male or female)
- LPS E.coli 0111:B4 (Sigma-Aldrich, catalog number: L2630-100MG )
- Dulbecco's phosphate-buffered saline (DPBS) (Corning, catalog number: 21-030-CV )
- XenoLight RediJect inflammation probe (PerkinElmer, catalog number: 760535 )
- Luminol sodium salt (Sigma-Aldrich, catalog number: A4685 )
- Isofluorane (Henry Schein, IsothesiaTM, catalog number: 10014450 )
- 5 mg/ml LPS (see Recipes)
- 20 mg/ml luminol sodium salt stock solution (see Recipes)
Equipment
- Anesthesia machine (VetEquip, model: 901808 ) or similar anesthesia equipment
- Rechargeable trimmer (Braintree Scientific, catalog number: VLP-323 75 )
- Scale (Kent Scientific, catalog number: SCL66110 )
- Biosafety cabinet
- IVIS spectrum (PerkinElmer, model: 124262 ) or a comparable luminescence imaging equipment
Software
- Living Image software (PerkinElmer)
Procedure
- Two days before the LPS intraperitoneal injection, place mice in the anesthesia machine and once the mice are anesthetized shave abdominal area with a trimmer, as fur quenches the luminescence signal (Figure 1).
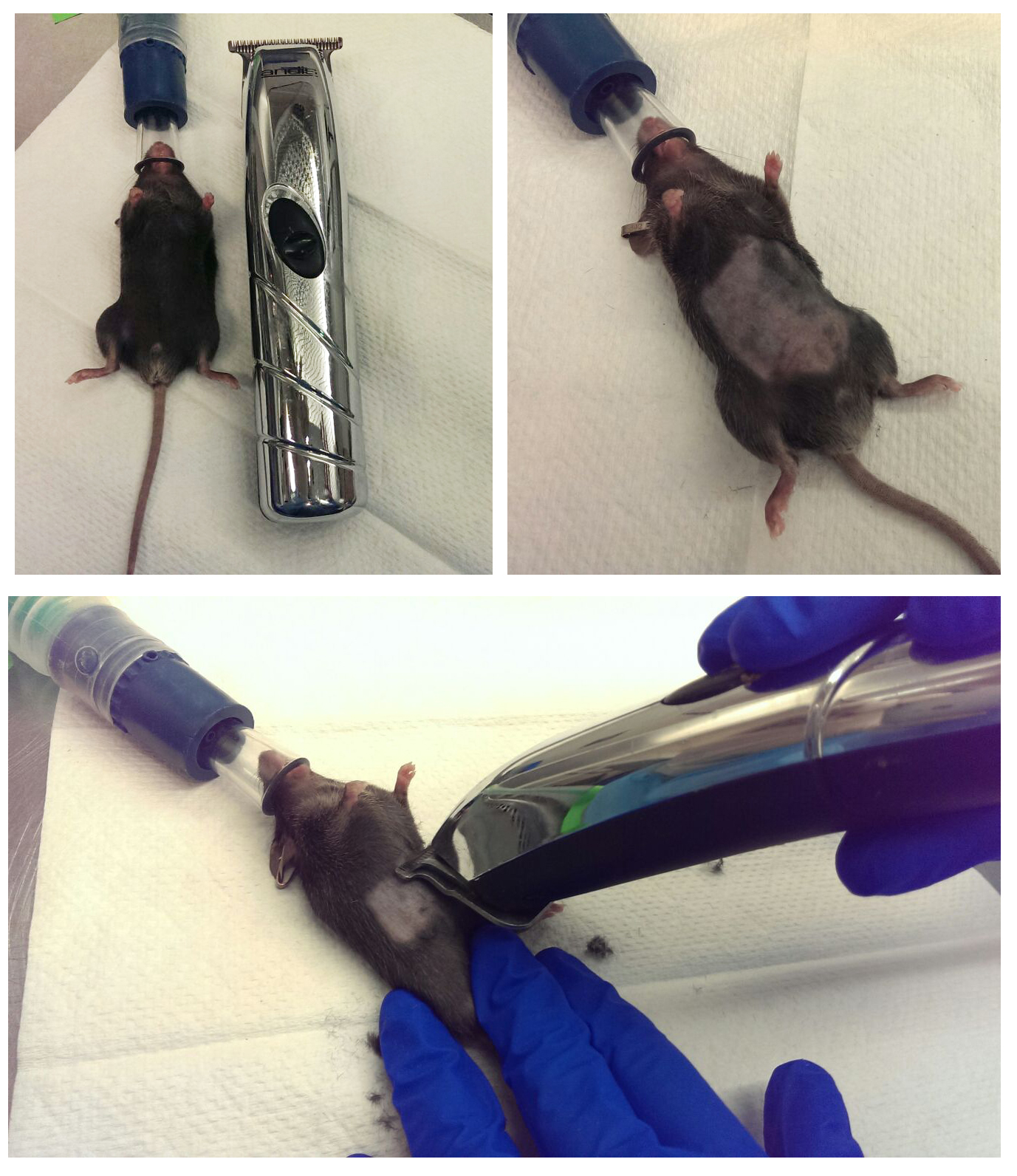
Figure 1. A representative picture of mice under anesthesia getting their abdominal area shaved with a trimmer - Weigh mice.
- In the day of the experiment dilute LPS in DPBS and prepare syringes for injection.
- Intraperitoneally inject mice with 2.5 mg/kg of LPS or the same volume DPBS for the control group (injection volume approximately 200 μl).
- 3 h later intraperitoneally inject mice with 200 mg/kg of XenoLight Rediject inflammation probe or 200 mg/kg luminol sodium salt (injection volume approximately 200 μl).
- Place mice in the IMPAC6 anesthesia chamber attached to the IVIS spectrum.
- Transfer mice to the IVIS spectrum and place mice abdomen facing up into the chamber and position each nose inside the cone that delivers the isofluorane (Figure 2).
- Start imaging anesthetized mice 10 min post XenoLight Rediject inflammation probe injection with a 5 min exposure capturing in vivo bioluminescence generated by the activity of MPO as a marker for infiltration of neutrophils. In order to imagine 5 mice select field of view D and select 1.5 cm subject height (Figures 2 and 3) (Gross et al., 2009; Tseng and Kung, 2012).
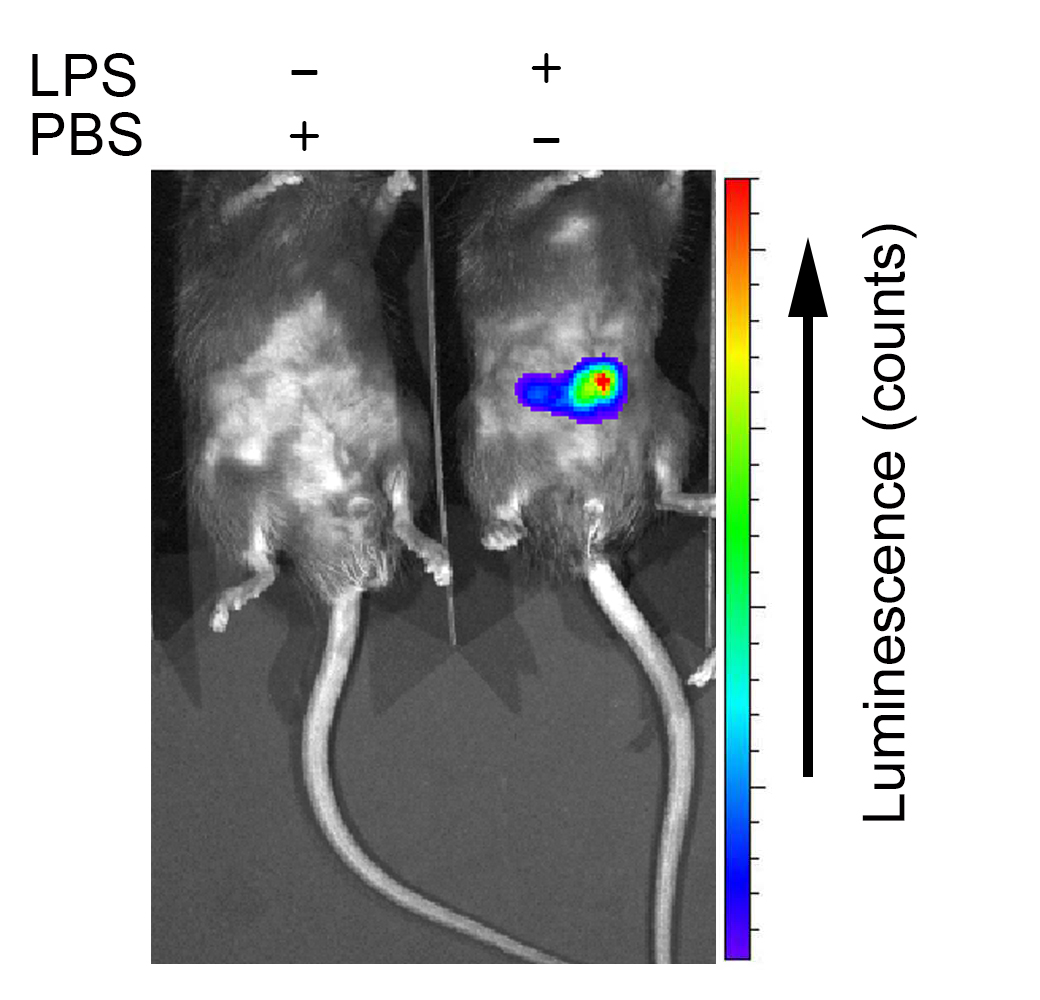
Figure 2. A representative example of in vivo imaging in 8 wk of age male C57BL/6 mice after i.p. injection of PBS (left) or LPS (2.5 mg/kg body weight) (right)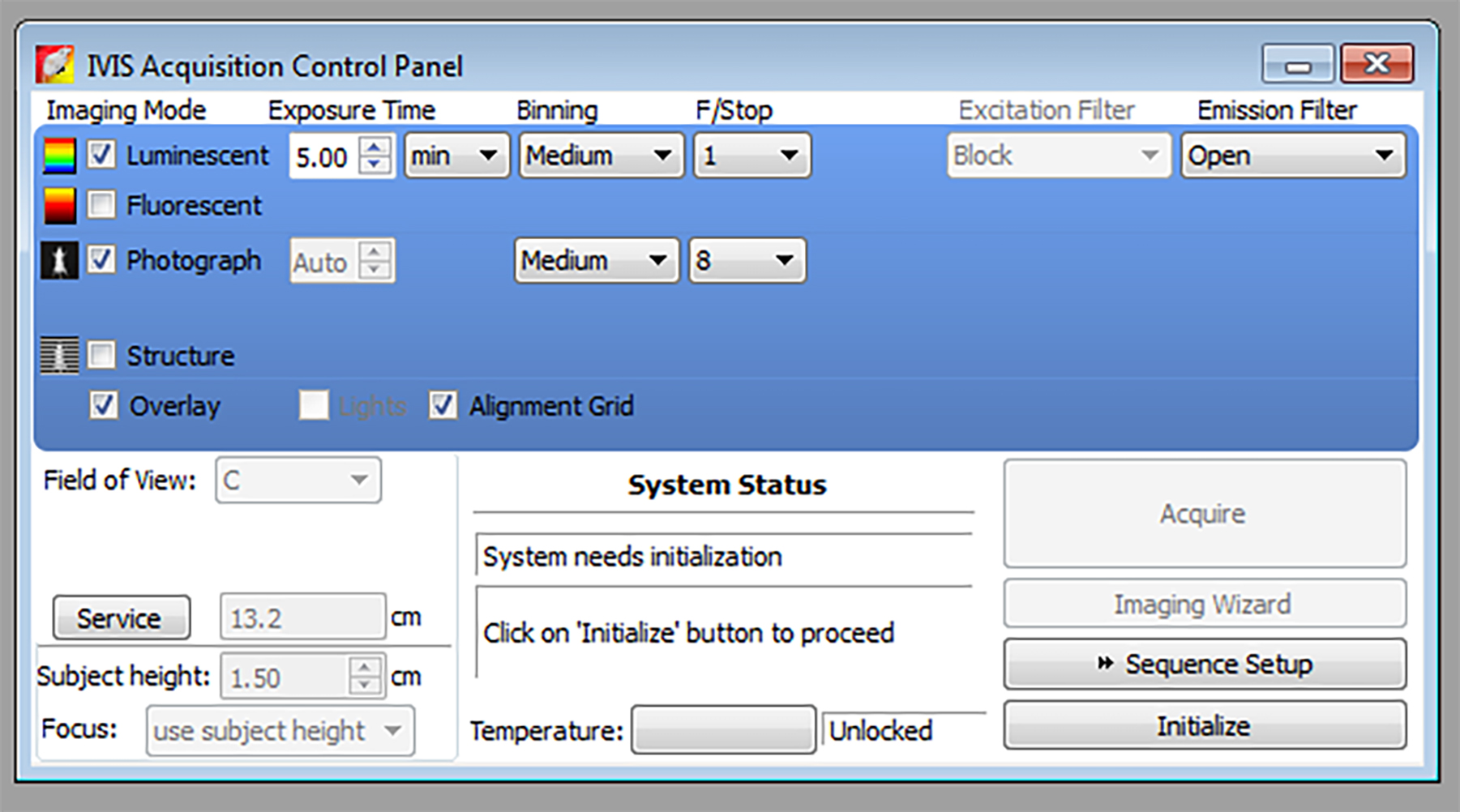
Figure 3. Screen capture image of the IVIS acquisition control panel - Quantify the MPO signal using the Living Image software. First select region of interest (ROI) using ROI tools and choose to automatically draw measurement ROIs and perform ROI analyses to measure photon radiance. Also measure background ROI and subtract from your ROI measurement. Use average radiance to plot your graph.
Recipes
- 5 mg/ml LPS
Dilute LPS in DPBS.
Filter sterillize with a 0.22 μm filter and aliquot stock solution at -80 °C. - 20 mg/ml luminol sodium salt stock solution
Prepare 20 mg/ml luminol sodium salt stock solution in DPBS.
Note: Luminol sodium salt for injection has to be prepared fresh each time in DPBS (20 mg/ml), filter sterillize with a 0.22 μm filter and protected from light until use.
Acknowledgments
This protocol was adapted from a previously published study (de Almeida et al., 2015). This work was supported by grants from the National Institutes of Health (AI099009 and AR064349 to C.S., AR066739 to A.D., AI120625 and AI120618 to C.S. and A.D., T32AR007611 to L.d.A., and the American Heart Association 13GRNT17110117 to C.S.).
References
- de Almeida, L., Khare, S., Misharin, A. V., Patel, R., Ratsimandresy, R. A., Wallin, M. C., Perlman, H., Greaves, D. R., Hoffman, H. M., Dorfleutner, A. and Stehlik, C. (2015). The PYRIN domain-only protein POP1 inhibits inflammasome assembly and ameliorates inflammatory disease. Immunity 43(2): 264-276.
- Gross, S., Gammon, S. T., Moss, B. L., Rauch, D., Harding, J., Heinecke, J. W., Ratner, L. and Piwnica-Worms, D. (2009). Bioluminescence imaging of myeloperoxidase activity in vivo. Nat Med 15(4): 455-461.
- Hagar, J. A., Powell, D. A., Aachoui, Y., Ernst, R. K. and Miao, E. A. (2013). Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science 341(6151): 1250-1253.
- Hoshino, K., Takeuchi, O., Kawai, T., Sanjo, H., Ogawa, T., Takeda, Y., Takeda, K. and Akira, S. (1999). Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol 162(7): 3749-3752.
- Kayagaki, N., Wong, M. T., Stowe, I. B., Ramani, S. R., Gonzalez, L. C., Akashi-Takamura, S., Miyake, K., Zhang, J., Lee, W. P., Muszynski, A., Forsberg, L. S., Carlson, R. W. and Dixit, V. M. (2013). Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341(6151): 1246-1249.
- McDonald, B., Pittman, K., Menezes, G. B., Hirota, S. A., Slaba, I., Waterhouse, C. C., Beck, P. L., Muruve, D. A. and Kubes, P. (2010). Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 330(6002): 362-366.
- Nagai, Y., Akashi, S., Nagafuku, M., Ogata, M., Iwakura, Y., Akira, S., Kitamura, T., Kosugi, A., Kimoto, M. and Miyake, K. (2002). Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat Immunol 3(7): 667-672.
- Park, B. S., Song, D. H., Kim, H. M., Choi, B. S., Lee, H. and Lee, J. O. (2009). The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 458(7242): 1191-1195.
- Poltorak, A., He, X., Smirnova, I., Liu, M. Y., Van Huffel, C., Du, X., Birdwell, D., Alejos, E., Silva, M., Galanos, C., Freudenberg, M., Ricciardi-Castagnoli, P., Layton, B. and Beutler, B. (1998). Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282(5396): 2085-2088.
- Ratsimandresy, R. A., Dorfleutner, A. and Stehlik, C. (2013). An update on PYRIN domain-containing pattern recognition receptors: from immunity to pathology. Front Immunol 4: 440.
- Shi, J., Zhao, Y., Wang, Y., Gao, W., Ding, J., Li, P., Hu, L. and Shao, F. (2014). Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514(7521): 187-192.
- Tseng, J. C. and Kung, A. L. (2012). In vivo imaging of inflammatory phagocytes. Chem Biol 19(9): 1199-1209.
- Whitfield, C. and Trent, M. S. (2014). Biosynthesis and export of bacterial lipopolysaccharides. Annu Rev Biochem 83: 99-128.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
de Almeida, L., Dorfleutner, A. and Stehlik, C. (2016). In vivo Analysis of Neutrophil Infiltration during LPS-induced Peritonitis. Bio-protocol 6(19): e1945. DOI: 10.21769/BioProtoc.1945.
Category
Immunology > Animal model > Mouse
Cell Biology > Cell movement > Cell migration
Cell Biology > Cell signaling > Stress response
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link