- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
15N-nitrate Uptake Activity and Root-to-shoot Transport Assay in Rice
Published: Vol 6, Iss 16, Aug 20, 2016 DOI: 10.21769/BioProtoc.1897 Views: 12578
Reviewed by: Tie LiuPooja SaxenaAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Visualization of Nitric Oxide, Measurement of Nitrosothiols Content, Activity of NOS and NR in Wheat Seedlings
Sandeep B. Adavi [...] Shailendra K. Jha
Oct 20, 2019 6353 Views

A Quick Method to Quantify Iron in Arabidopsis Seedlings
Chandan Kumar Gautam [...] Wolfgang Schmidt
Mar 5, 2022 3931 Views

CAPS-Based SNP Genotyping for Nitrogen-Response Phenotypes in Maize Hybrids
Jannis Jacobs [...] Peter K. Lundquist
Dec 20, 2025 550 Views
Abstract
15N is a nonradioactive heavy isotope of nitrogen, widely used for biochemical and physiological research in plants. For instance, 15N-KNO3 was used as the nitrogen source in plants in order to investigate nitrate uptake activity and transport from roots to shoots (Lin et al., 2008). Here, we describe a detailed pipeline used for labeling living rice (Oryza sativa) plants with 15N-KNO3 and determination of net nitrate uptake and transport activity, and this protocol was proved to be valid in Arabidopsis and rice (Lin et al., 2008; Hu et al., 2015).
Keywords: Nitrate uptakeMaterials and Reagents
- 96-well plate
- Rice seeds (Zhonghua11, ZH11)
- NaClO [Sinopharm Chemical Reagent Co.,Ltd (SCRC), catalog number: 7681-52-9 ]
- KNO3 (SCRC, catalog number: 7757-79-1 )
- K15NO3 (Sigma-Aldrich, catalog number: 57654-83-8 )
- CaCl2 (SCRC, catalog number: 10043-52-4 )
- MgSO4·7H2O (SCRC, catalog number: 10034-99-8 )
- KH2PO4 (SCRC, catalog number: 7778-77-0 )
- FeSO4·7H2O (SCRC, catalog number: 7782-63-0 )
- EDTA-Na2 (SCRC, catalog number: 6381-92-6 )
- NaSiO3·9H2O (SCRC, catalog number: 13517-24-3 )
- H3BO3 (SCRC, catalog number: 10043-35-3 )
- CuSO4·5H2O (SCRC, catalog number: 7758-99-8 )
- ZnSO4·7H2O (SCRC, catalog number: 7446-20-0 )
- MnCl2·4H2O (SCRC, catalog number: 13446-34-9 )
- Na2MoO4·2H2O (SCRC, catalog number: 10102-40-6 )
- CaSO4·2H2O (SCRC, catalog number: 10101-41-4 )
- Modified Kimura B solution (see Recipes)
- 5 mM 15N-KNO3 (see Recipes)
- 0.1 mM CaSO4 solution (see Recipes)
Equipment
- Growth chamber (SANYO, model: MLR-351H )
- Isotope ratio mass spectrometer(Thermo Fisher Scientific, model: Finnigan Delta Plus XP) with elemental analyzer (Thermo Fisher Scientific, model: Flash EA 1112 )
Procedure
- Seed germination
Rice seeds are surface-sterilized with 2.5% sodium hypochlorite (NaClO) for 30 min and then soaked in tap water, put in an incubator chamber at 37 °C for 2 days (d), change water every 12 h till the seeds germinate. - Seedling growth
Uniformly germinated seeds are selected and put into 96-well plates, then transferred to clear water until roots length reach 3 cm, after which transplant the seeds to modified Kimura B solution. Rice seedlings are grown in a growth chamber with a 12-h light (30 °C)/12-h dark (28 °C) photoperiod and 70% humidity for about 2 weeks. The solution is changed every day. - 15N-nitrate uptake assay
After 2-week cultivation, rice seedlings are pretreated with modified Kimura B solution for 2 h, after which the rice roots are washed by tap water twice and rice seedlings are transferred to modified Kimura B solution containing 5 mM 15N-KNO3 for 3 h. - Rice seedlings harvest
After 3 h absorption, rice roots are rinsed with 0.1 mM CaSO4 for 2 min to remove the 15N-NO3- on the root surface, then roots and shoots are harvested separately and dried at 70 °C to constant weight in paper bags. Dried samples are ground to fine powder in mortars for subsequent assay. - Calculation of nitrogen uptake
About 0.5 mg dried powder is analyzed by isotope ratio mass spectrometer and the data of 15N content are obtained (Brand, 1996). While detecting the 15N-nitrate uptake activity, a formula [total 15N amount of whole plant (TN)/dry weight (DW) of root (DWR)/3 h] is applied to the calculation, i.e., the amount of 15N take up per unit weight of roots per unit time, total 15N amount of whole plant is derived from the sum of N amount of shoots and roots. The ratio of shoot 15N content (SN) to root 15N content (RN) is used to represent the root-to-shoot transport activity (the higher the value, the higher root-to-shoot transport activity).
Representative data

Figure 1. A 96-well plate that was cut off the bottom well-suited for the growth of rice seedlings. After being put into the 96-well plate, rice roots could grow downward into solution underneath while rice shoots could grow upward tidily along each well.
Figure 2. 10-day-old rice seedlings grown on a 96-well plate. The container under the 96-well plate is full of modified Kimura B solution, usually the container is wrapped with light-tight material (e.g., tinfoil) to protect rice roots from light.
Table1. 15N content of shoots and roots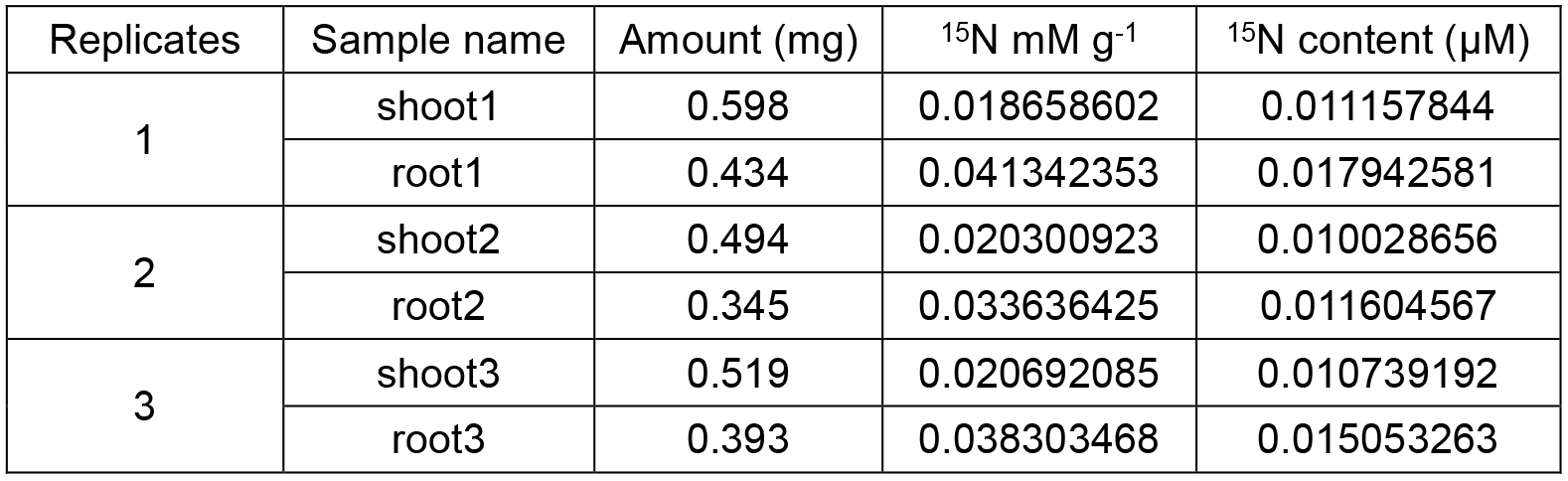
Table2. Calculation of 15N-nitrate uptake activity and root-to-shoot transport activity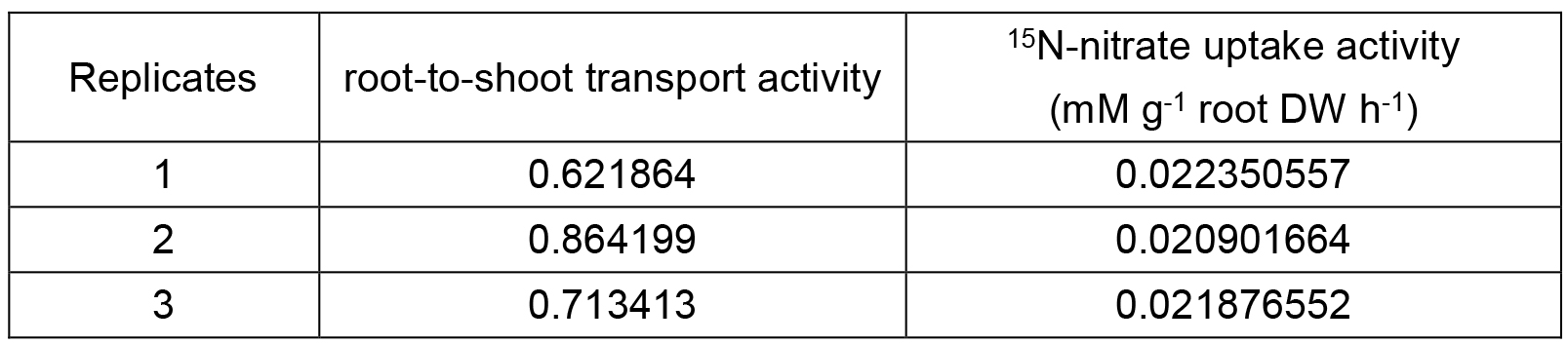
Notes
- Uniformly germinated seeds were selected to make sure the same growing status of different lines.
- The germinated seeds could be transferred to modified Kimura B solution until seminal root length was about 3 to 5 cm.
- The aim of pretreating rice seedlings with modified Kimura B solution for 2 h is to make sure the rice seedlings could get back to a relatively normal physiological state before the 15N-nitrate uptake assay.
Recipes
- Modified Kimura B solution
5 mM KNO3
0.36 mM CaCl2
0.54 mM MgSO4
0.18 mM KH2PO4
40 µM FeSO4-EDTA
18.8 µM H3BO3
13.4 µM MnCl2
0.32 µM CuSO4
0.3 µM ZnSO4
0.03 µM Na2MoO4
1.6 mM Na2SiO3
pH 6.0 - 5 mM 15N-KNO3
As mentioned in Procedure 3, 5 mM 15N-KNO3 was used to replace 5 mM KNO3 in modified Kimura B solution while other ingredients remain unchanged. - 0.1 mM CaSO4 solution
Dissolve 0.0172 g CaSO4·2H2O in 1 L deionized water
Acknowledgments
The hydroponic culture has been illuminated in Lin et al. (2008) and Hu et al. (2015), the 15N-nitrate uptake activity and root-to-shoot transport activity assay was cited from Hu et al. (2015). This work was supported by grants from the Ministry of Science and Technology of China (2014AA10A602-5, 2015CB755702), and the Chinese Academy of Sciences (XDA08010400).
References
- Brand, W. A. (1996). High precision isotope ratio monitoring techniques in mass spectrometry. Journal of Mass Spectrometry 31:225-235.
- Delhon, P., Gojon, A., Tillard, P. and Passama, L. (1995). Diurnal regulation of NO3- uptake in soybean plants I. changes in NO3- influx, efflux, and N utilization in the plant during the day-night cycle. Journal of Experimental Botany 46(291): 1585-1594.
- Hu, B., Wang, W., Ou, S. J., Tang, J. Y., Li, H., Che, R. H., Zhang, Z. H., Chai, X. Y., Wang, H. R., Wang, Y. Q., Liang, C. Z., Liu, L. C., Piao, Z. Z., Deng, Q. Y., Deng, K., Xu, C., Liang, Y., Zhang, L. H., Li, L. G. and Chu, C. C. (2015). Variation in NRT1.1B contributes to nitrate-use divergence between rice subspecies. Nature Genetics 47(7): 834-838.
- Lin, S. H., Kuo, H. F., Canivenc, G., Lin, C. S., Lepetit, M., Hsu, P. K., Tillard, P., Lin, H. L., Wang, Y. Y., Tsai, C. B., Gojon, A. and Tsay, Y. F. (2008). Mutation of the Arabidopsis NRT1.5 nitrate transporter causes defective root-to-shoot nitrate transport. Plant Cell 20(9): 2514-2528.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Liu, Y., Hu, B. and Chu, C. (2016). 15N-nitrate Uptake Activity and Root-to-shoot Transport Assay in Rice. Bio-protocol 6(16): e1897. DOI: 10.21769/BioProtoc.1897.
Category
Plant Science > Plant physiology > Nutrition
Share
Bluesky
X
Copy link










