- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A Highly Efficient Method for Measuring Oxygen Consumption Rate in Fusarium graminearum
Published: Vol 6, Iss 15, Aug 5, 2016 DOI: 10.21769/BioProtoc.1887 Views: 10903
Reviewed by: Valentine V TrotterEmilia Krypotou Jose Thekkiniath

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Quantification of Respiratory Activity in Biofilms
Cláudia N. H. Marques and Scott A. Craver
Sep 20, 2015 10797 Views

High Resolution Respirometry in Candida albicans
Lucian Duvenage [...] Campbell W. Gourlay
Sep 5, 2019 4984 Views
Abstract
The filamentous ascomycete Fusarium graminearum is the causal agent of Fusarium head blight, a devastating disease of cereals with a worldwide distribution. Fusarium graminearum infections result in a quantitative yield reduction by impairing the growth of the kernels, and a qualitative reduction by poisoning the remaining kernels with mycotoxins toxic to animals and humans. The colonization of wheat florets by phytopathogenic fungus requires high-efficiency energy generation in the mitochondria (Bönnighausen et al., 2015). Mitochondrial activity in microorganisms can be measured using the oxygen consumption rate (OCR) method. Here we describe a method for the assessment of fungal respiration using an XF24 extracellular flux analyzer. The Seahorse XF Analyzer is a microplate-based respirometer which measures oxygen consumption by changes in the fluorescence of immobilized fluorophores (Gerencser et al., 2009). Multiple mitochondrial parameters can be measured by the application of mitochondrial substrates and inhibitors which are injected automatically during the assays via ports (Divakaruni et al., 2014). The experimental work-flow involves the inoculation with conidia and the application of specific inhibitors of mitochondrial functions. The analysis of fungal respiration represents a valuable tool that complements classical phenotypic screenings.
Materials and Reagents
- Pipette tips
- Conidia of F. graminearum (preferably fresh, not frozen)
- Seahorse XF Cell Mito Stress Test Kit including oligomycin, rotenone, antimycin A and Cyanide-p-trifluoromethoxyphenyl hydrazone (Seahorse Bioscience, catalog number: 103015-100 )
- Seahorse XF24 Islet FluxPak containing XF24 cartridges and Islet capture microplates (Seahorse Bioscience, catalog number: 101174-100 )
- Calibrant solution (Seahorse Bioscience, catalog number: 103059-000 )
- Ca(NO3)2·4H2O (Carl Roth, catalog number: X886.1 )
- KH2PO4 (Carl Roth, catalog number: P018.1 )
- MgSO4·7H2O (Sigma-Aldrich, catalog number: 230391-500G )
- NaCl (Carl Roth, catalog number: 9265.1 )
- Sucrose
- H3BO3 (Carl Roth, catalog number: 6943.1 )
- CuSO4·5H2O (Sigma-Aldrich, catalog number: 209198-250G )
- KI (Carl Roth, catalog number: 6750.1 )
- MnSO4·H2O (Carl Roth, catalog number: 7347.2 )
- (NH4)6Mo7O24·4H2O (Sigma-Aldrich, catalog number: 09880-100G )
- ZnSO4·7H2O (Carl Roth, catalog number: 7316.1 )
- FeCl3·6H2O (Carl Roth, catalog number: 7119.1 )
- Solution A (see Recipes)
- Solution B (see Recipes)
- Suspension D (see Recipes)
- Minimal medium (see Recipes)
Equipment
- Multi-channel pipettes (Sigma-Aldrich, catalog number: Z683930-1EA )
- XF24 extracellular flux analyzer (Seahorse Bioscience, model: Seahorse XFe24 )
- Incubator at 28 °C (Heraeus B20/UB20) (Thermo Fisher Scientific, catalog number: 50061005 )
- Microplate centrifuge (Eppendorf, catalog number: 022620509 )
Software
- XF24 Extracellular Flux Analyzer Software
- Spreadsheet software program [e.g., Excel (Microsoft)]
Procedure
All steps of OCR measurement are summarized in Figure 1. This section describes the setup of an appropriate program on the Seahorse controller. The injection compounds will be injected subsequently in all wells by air pressure.
- Program an equilibration step of 35 min.
- Measure OCRs
- Program three measuring cycles: 2 min mixing, 2 min delay, 3 min measurement.
- Optional: use injection port A to inject 50 µl of a test substance, e.g., gamma amino (5 mM final concentration) (Bönnighausen et al., 2015).
- After injection of test substance perform 20 measuring cycles (2 min mixing, 2 min delay, 3 min measurement).
- Use injection port B to inject oligomycin (55 μl, 4 μM final concentration). Oligomycin is used to estimate the amount of oxygen that is consumed for ATP synthesis.
- Immediately perform 3-6 measuring cycles (2 min mixing, 2 min delay, 3 min measurement).
- Use injection port C to inject Cyanide-p-trifluoromethoxyphenyl hydrazone (FCCP) (60 μl, 4 μM final concentration). FCCP uncouples the respiratory chain. Its application allows estimation of the spare respiratory capacity.
- Immediately perform 3-6 measuring cycles (2 min mixing, 2 min delay, 3 min measurement)
- Use injection port D to inject rotenone and antimycin A (65 μl, 4 μM final concentration). Rotenone and antimycin A inhibit complex I and II of the mitochondrial electron transport chain, respectively, and, thereby, completely deplete mitochondrial respiration.
- Immediately perform 3-6 measuring cycles (2 min mixing, 2 min delay, 3 min measurement).
Figure 1. Workflow for oxygen consumption rate measurement in Fusarium graminearum
Preparations on the day before the assay - Program three measuring cycles: 2 min mixing, 2 min delay, 3 min measurement.
- Hydrate the cartridges according to the manufacturer’s instructions with calibrant 24 h prior to assay in an incubator at 28 °C, turn the XF24 analyzer on, start the XF24 software, and allow the instrument to get a stable temperature of 28 °C.
- Insert the capture screens with the capture screen insert tool into the XF24 Islet capture microplate.
- Add 450 µl freshly prepared minimal medium into each well of the XF24 Islet capture microplate.
- Add 5,000 conidia (50 µl of a 100 conidia µl-1 suspension in water) into an XF24 Islet capture microplate, leave two wells blank for background subtraction.
- Centrifuge the plate for 2 min at 1,000 x g at room temperature to get all conidia below the capture screen.
- Incubate for 24 h at 28 °C (optimal growth temperature for F. graminearum).
Workflow on the day of the assay - Check for conidia germination under the dissecting microscope (Figure 2).
- Exchange the media with fresh minimal medium by taking up the liquid above the capture screen with a pipette and replacing it with fresh minimal medium (500 µl at room temperature).
- Incubate the plate for 1 h at 28 °C to allow the mycelia adjust to the new medium. Longer incubation might lead to nitrogen depletion in the medium due to uptake by the growing fungus.
- Load the injection ports of the cartridge as predefined in the program section and then follow the instructions of the XF24 controller to start the calibration of the hydrated cartridge.
- Insert the Islet microplate into XF24 extracellular flux analyzer and start the run.
- Analyze data according to the XF24 Seahorse Software and Operation Manual and a spreadsheet software program.
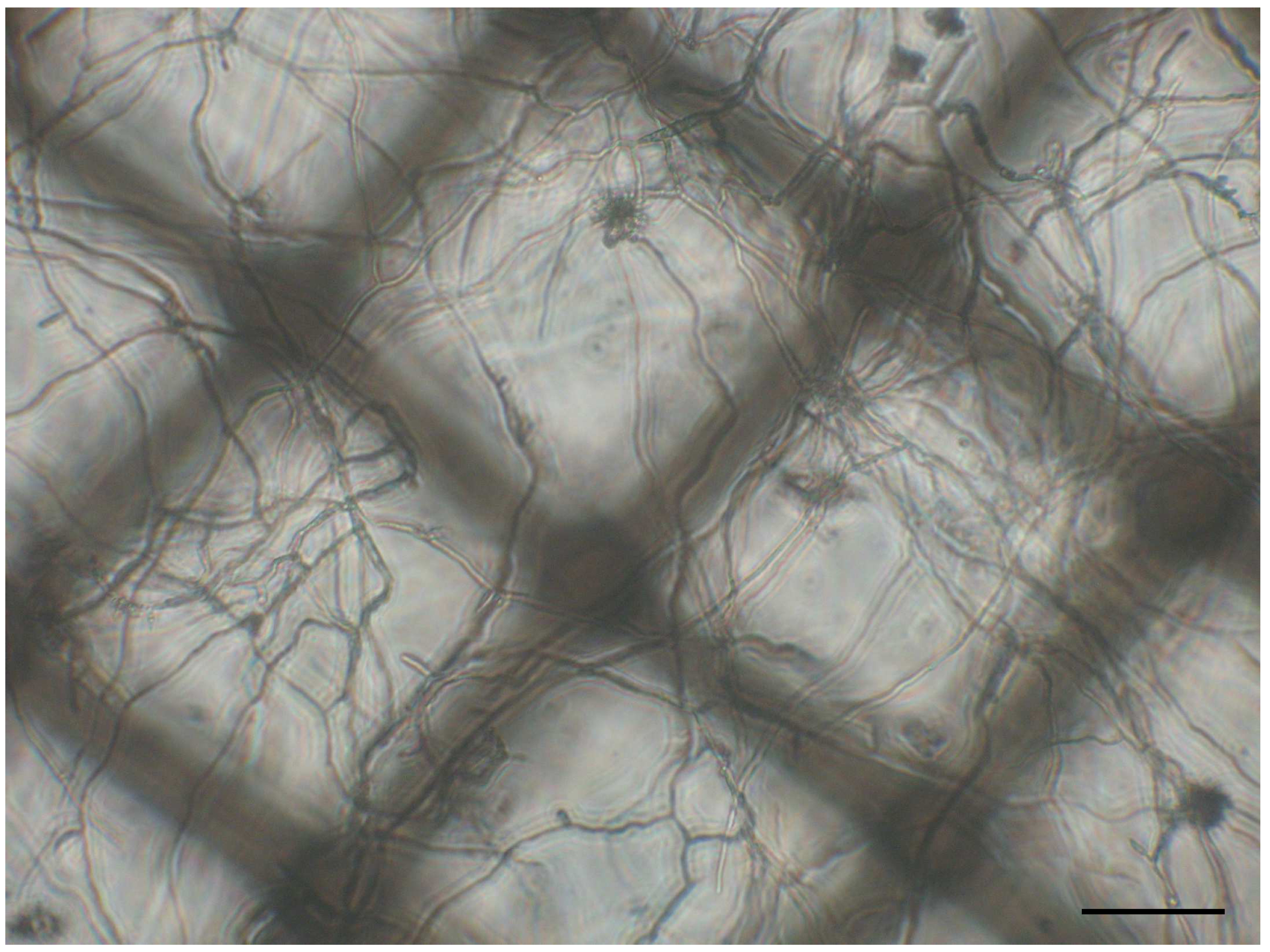
Figure 2. Fungal mycelia of Fusarium graminearum. An XF24 Islet capture microplate filled with 450 µl minimal medium was inoculated with 5,000 conidia and incubated for 24 h at 28 °C. Scale bar: 100 µm
Representative data
A measurement of fungal respiration according to the procedures described above should typically result in an OCR as depicted in Figure 3. We recommend 5 replicates per experimental group.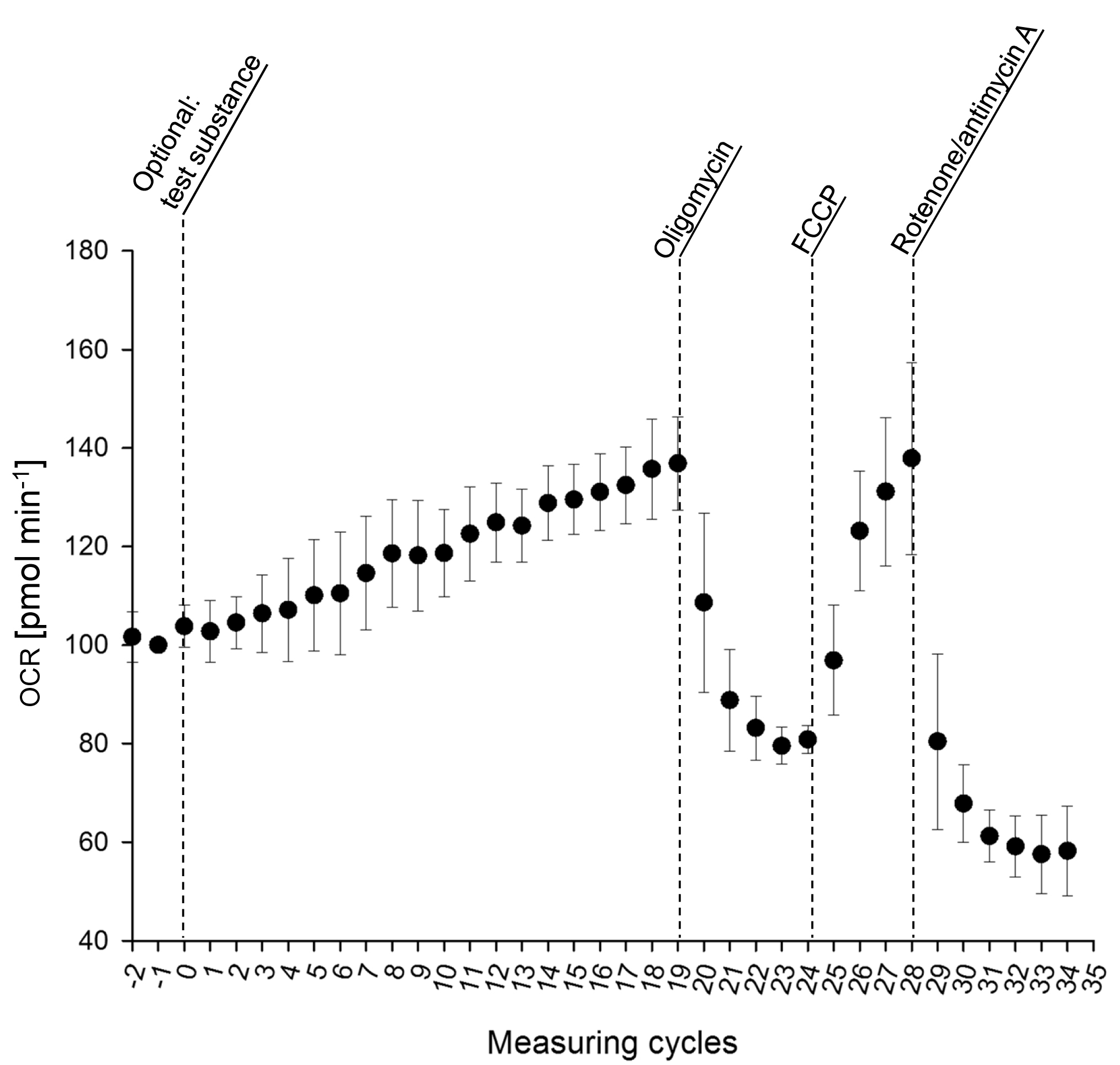
Figure 3. Oxygen consumption rate of mycelia of Fusarium graminearum grown in liquid minimal medium
Notes
The measurement of oxygen consumption rates in axenic culture described here was highly reproducible.
Recipes
- Solution A
100 g/L Ca(NO3)2·4H2O - Solution B
20 g/L KH2PO4
25 g/L MgSO4·7H2O
10 g/L NaCl, sterilized by filtration - Suspension D
60 g/L H3BO3
390 mg/L CuSO4·5H2O
13 mg/L KI
60 mg/L MnSO4·H2O
51 mg/L (NH4)6Mo7O24·4H2O
5.48 g/L ZnSO4·7H2O
932 mg/L FeCl3·6H2O - Minimal medium (1 L)
10 ml of solution A
10 ml of solution B
10 g sucrose
1 ml of suspension D
Acknowledgments
The authors thank the “Kompetenzzentrum Nachhaltige Universität” of the University Hamburg for funding.
References
- Bönnighausen, J., Gebhard, D., Kröger, C., Hadeler, B., Tumforde, T., Lieberei, R., Bergemann, J., Schäfer, W. and Bormann, J. (2015). Disruption of the GABA shunt affects mitochondrial respiration and virulence in the cereal pathogen Fusarium graminearum. Molecular Microbiology 98(6): 1115-1132.
- Gerencser, A. A., Neilson, A., Choi, S. W., Edman, U., Yadava, N., Oh, R. J., Ferrick, D. A., Nicholls, D. G. and Brand, M. D. (2009). Quantitative microplate-based respirometry with correction for oxygen diffusion. Anal Chem 81(16): 6868-6878.
- Divakaruni, A. S., Paradyse, A., Ferrick, D. A., Murphy, A. N. and Jastroch, M. (2014). Analysis and interpretation of microplate-based oxygen consumption and pH data. Methods Enzymol 547: 309-354.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Gebhard, D., Bönnighausen, J., Bergemann, J., Schäfer, W. and Bormann, J. (2016). A Highly Efficient Method for Measuring Oxygen Consumption Rate in Fusarium graminearum. Bio-protocol 6(15): e1887. DOI: 10.21769/BioProtoc.1887.
Category
Microbiology > Microbial physiology > Respiration
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










