- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Retinal Differentiation of Mouse Embryonic Stem Cells
Published: Vol 6, Iss 13, Jul 5, 2016 DOI: 10.21769/BioProtoc.1851 Views: 15831
Reviewed by: Hui ZhuPatrick Ovando-RocheAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Isolation of Neural Stem Cells from the Embryonic Mouse Hippocampus for in vitro Growth or Engraftment into a Host Tissue
Oksana Rybachuk [...] Vitaliy Kyryk
Feb 20, 2019 10086 Views

Generation of Mouse Primitive Endoderm Stem Cells
Yasuhide Ohinata [...] Haruhiko Koseki
Nov 20, 2023 2452 Views

An Efficient Method for Immortalizing Mouse Embryonic Fibroblasts by CRISPR-mediated Deletion of the Tp53 Gene
Srisathya Srinivasan and Hsin-Yi Henry Ho
Jan 20, 2025 2758 Views
Abstract
Groundbreaking studies from Dr. Yoshiki Sasai’s laboratory have recently introduced novel methods to differentiate mouse and human Embryonic Stem Cells (mESCs and hESCs) into organ-like 3D structures aimed to recapitulate developmental organogenesis programs (Eiraku et al., 2011; Eiraku and Sasai, 2012; Nakano et al., 2012; Kamiya et al., 2011). We took advantage of this method to optimize a 3D protocol to efficiently generate retinal progenitor cells and subsequently retinal neurons in vitro. This culture system provides an invaluable platform both to study early developmental processes and to obtain retinal neurons for transplantation approaches. The protocol described here has been successfully applied to several mouse ESC (including the R1, WD44 and G4 cell lines) and mouse induced-Pluripotent Stem Cell (iPSCs) lines.
Keywords: RetinaMaterials and Reagents
- 96-well ultra low-cell-adhesion plate, Lipidure-Coat (Amsbio LLC, catalog number: AMS.51011610 ) or PrimeSurface 96U plate (Sumitomo Bakelite, catalog number: MS-9096U )
- 6-well ultra low-cell-adhesion plate (Thermo Fisher Scientific, CorningTM, catalog number: 07-200-601 )
- 100 mm cell culture dish (Corning, BD Falcon®, catalog number: 353003 )
- Glass Pasteur pipette
- Mouse Embryonic Stem Cells (mESCs) or mouse Induced-Pluripotent Stem Cells (miPSCs)
- Phosphate Buffer Saline (PBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 10010-023 )
- Water, cell culture grade (Thermo Fisher Scientific, catalog number: 15230-162 )
- Dulbecco’s Modified Eagle Medium (DMEM) high glucose (Thermo Fisher Scientific, GibcoTM, catalog number: 10564-011 )
- Glasgow’s MEM (GMEM) (Thermo Fisher Scientific, GibcoTM, catalog number: 11710-035 )
- Dulbecco’s modified Eagle’s medium/nutrient F-12 Ham (DMEM/F12 Ham) (Thermo Fisher Scientific, GibcoTM, catalog number: 10565-018 )
- Fetal Bovine Serum (FBS), ESC Qualified (Thermo Fisher Scientific, catalog number: 10439-024 )
- Knock-Out Serum Replacement (KSR) (Thermo Fisher Scientific, GibcoTM, catalog number: 10828-028 )
- Mixture of penicillin and streptomycin (Pen/Strep) (Thermo Fisher Scientific, GibcoTM, catalog number: 15140-122 )
- Leukemia Inhibitory Factor (LIF) (Merck Millipore Corporation, catalog number: ESG1107 )
- GSK3b inhibitor (Stemolecule CHIR99021) (Stemgent, catalog number: 04-0004-02 )
- MAPK/ERK inhibitor (Stemolecule PD0325901) (Stemgent, catalog number: 04-0006-02 )
- TrypLE Trypsin Replacement (Thermo Fisher Scientific, catalog number: 12605-028 )
- 10 mM non-essential amino acids (NEAA) solution (Thermo Fisher Scientific, GibcoTM, catalog number: 11140-050 )
- Sodium pyruvate solution (Thermo Fisher Scientific, GibcoTM, catalog number: 11360-070 )
- 2-mercaptoethanol (2-ME) (Sigma-Aldrich, catalog number: M7522 )
- Growth factor reduced Matrigel® (GFR Matrigel) (Corining®, catalog number: 356230 )
- B27 supplement (Thermo Fisher Scientific, GibcoTM, catalog number: 17504044 )
- N2 supplement (Thermo Fisher Scientific, GibcoTM, catalog number: 17502-048 )
- All-trans retinoic acid (Sigma-Aldrich, catalog number: R2625-50MG )
- Taurine (Sigma-Aldrich, catalog number: T-8691 )
- MEF medium (see Recipes)
- LIF + 2i medium (see Recipes)
- Retinal Differentiation medium (RD medium) (see Recipes)
- Tom’s medium (see Recipes)
Equipment
- Incubator (37 °C and 5% CO2) (Thermo Scientific, model: HERACell 150i )
- Centrifuge (Eppendorf, model: Conical centrifuge 5702 )
- Tissue culture hood, Type B2 Biological Safety Cabinet (Thermo Scientific, model: 1300 Series Class II )
- Hemocytometer (Neubauer chamber) or equivalent method
- Inverted microscope (Leica, model: DMi8 )
Procedure
- Coating plates
- Thaw an aliquot of Growth Factor Reduced-Matrigel by submerging the tube in ice for 1 h.
- Dilute the Matrigel 1:50 in cold MEF medium and transfer the media and the Matrigel to a TC dish. For 6-well plates, we use 1 ml/well and 7 ml for 10 cm plates.
- Incubate at 37 ºC for at least 1 h.
- Maintenance of mESCs
- mESCs are plated on a 10 cm dish pre-coated with Growth Factor Reduced-Matrigel, in LIF + 2i medium.
- mESCs are next incubated in a 5% CO2 incubator at 37 ºC until passage or retinal differentiation.
- Medium is changed every other day.
Note: Special attention should be taken to avoid overconfluency of mESCs in maintenance cultures as it might result in detrimental differentiation of the cells.
- mESCs are plated on a 10 cm dish pre-coated with Growth Factor Reduced-Matrigel, in LIF + 2i medium.
- Generation of Retinal Cells from undifferentiated mESCs
- Remove the media from a 50% confluent plate of undifferentiated mESC (at least four passages off feeder layers, Figure1) and add TrypLE Trypsin replacement to the cells. Place the cells back in the incubator for 3-5 min until the mESCs colonies start to lift. Since TrypLE Trypsin replacement is not inhibited by the serum in the media, this method does not require a pre-wash with PBS.
- Add 5 ml of MEF media to the plate and gently lift off the cells using a glass Pasteur pipette. Observe that the cells are removed from the plate and pipette up and down to achieve a single cell suspension being careful to avoid bubbles.

Figure 1. Typical morphologies of undifferentiated mESCs (Day 0). Feeder-free undifferentiated R1 mESCs (originally generated by Andras Nagy) are shown. Scale bars: 500 μm (A) and 250 μm (B). - mESC cells are pelleted at 190 x g for 5 min.
- Carefully disperse the pellet using 5 ml of Retinal Differentiation medium (RD medium).
- Count the cell density using a hemocytometer (Neubauer chamber) or equivalent method.
- Plate 3,000 cells per 100 μl in a 96-well low attachment plate (100 μl/well) in RD medium. Cell aggregates (Embryoid Bodies) should form in less than 12 h. The day on which the culture is started is designated as Day 0.
- 24 h later (Day 1), add Growth Factor Reduced-Matrigel to a final concentration of 2% (vol/vol). The lot-to-lot variability in commercial Matrigel products can change the efficiency of retinal induction; we preferentially use one of relatively high concentration (> 9.5 mg/ml). We recommend diluting the Matrigel 1:10 in cold RD medium and add 20 μl of the dilution/well.
- On Day 3 a clear layer of neuroepithelial cells is apparent (Figure 2).
Note: Different cell lines might grow at different rates and consequently the initial number of cells per well might need to be adjusted. We found that for most of the lines tested 3,000-5,000 cells/well was the optimal cell density.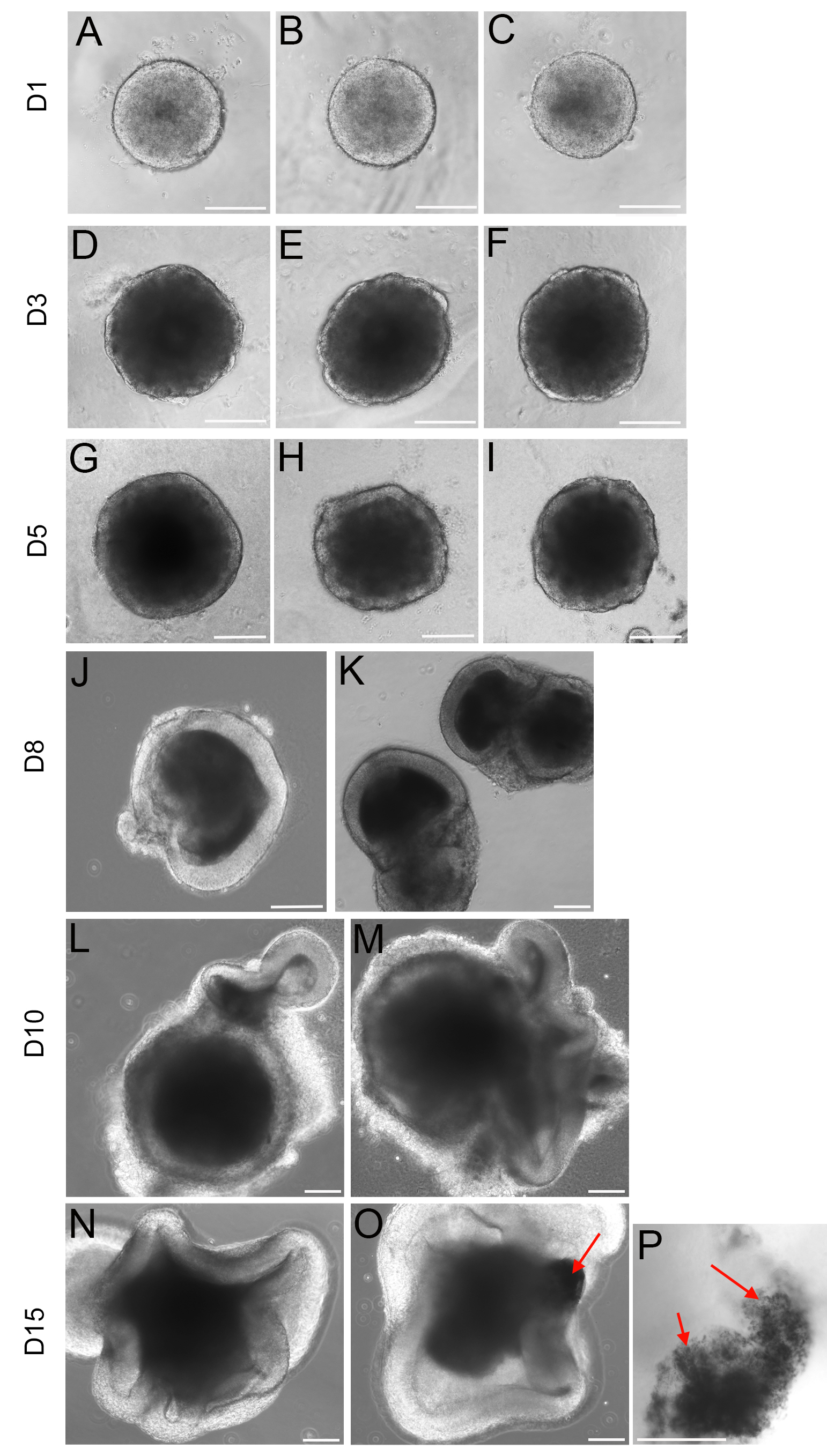
Figure 2. Typical morphologies of embryoid bodies at the different differentiation stages. R1 mESCs were differentiated using the method described here. Around each EB (embryoid body) a clear neuroepithelial layer develops from Day 3 (D-F), and from Day 5 this layer is conspicuous (G-K), optic vesicle and optic cup-like structures are apparent from day 7-8 (L-O). Scale bars: 150 μm.
Note: Retinal Pigmented Epithelium (RPE) cells are observed after Day 13 (red arrows in O and P).
- Remove the media from a 50% confluent plate of undifferentiated mESC (at least four passages off feeder layers, Figure1) and add TrypLE Trypsin replacement to the cells. Place the cells back in the incubator for 3-5 min until the mESCs colonies start to lift. Since TrypLE Trypsin replacement is not inhibited by the serum in the media, this method does not require a pre-wash with PBS.
- Retinal maturation
- On Day 7, the embryoid bodies (EBs) are transferred to 6-well low-attachment plates in Tom’s medium supplemented with B27 and N2 to promote neuronal differentiation. From Day 7 medium should be changed every other day.
- From Day 10 to Day 15, in addition to B27 and N2, Tom’s medium is also supplemented with 0.5 μM retinoic acid and 1 mM taurine. RPE cells are apparent from Day 13 (Figure 2)
- From Day 15 onwards, EBs are maintained in Tom’s medium. Rhodopsin+ Rod photoreceptors are detected from Day 24 and after Day 35, the EBs exhibit all the layers of the mature retina.
- On Day 7, the embryoid bodies (EBs) are transferred to 6-well low-attachment plates in Tom’s medium supplemented with B27 and N2 to promote neuronal differentiation. From Day 7 medium should be changed every other day.
Recipes
- MEF medium (100 ml)
89 ml DMEM
10 ml FBS
1 ml Pen/Strep - LIF + 2i medium (100 ml)
90 ml of MEF medium
10 ml FBS
1 ml sodium pyruvate
100 μl 2-ME (Stock 0.1 M)
100 μl LIF (Stock 10 millions units/Ml)
On the day of use, supplement with 3 µM of GSK3β inhibitor Stemolecule CHIR99021 and 0.4 µM of MEK inhibitor Stemolecule PD0325901 - 2-ME (monthly preparation)
35 µl 2-ME
5 ml of distilled water - Retinal Differentiation (RD) medium (100 ml)
96.4 ml Glasgow minimum essential medium (GMEM)
1.5 ml KSR
1 ml non-essential amino acids (stock 100x)
1 ml sodium pyruvate (stock 100x)
100 µl 2-ME (stock 0.1 M) - Tom’s medium (100 ml)
98 ml DMEM: F12
1 ml non-essential amino acids (stock 100x)
1 ml sodium pyruvate (stock 100x)
- From Day 7 to Day 15:
1 ml N2 (stock 100x)
2 ml B27 (stock 50x)
- From Day 10 to Day 15:
0.5 μM retinoic acid (stock 1 mM)
1 mM taurine (stock 1 M)
Acknowledgments
This protocol was originally published as part of: La Torre et al. (2015). The author wishes to thank all present and past members of the Reh and Bermingham-McDonogh laboratories for many helpful discussions. Special thanks to Tom Reh and Akina Hoshino for invaluable help and advice, and NIH 1 PO1 GM081619 and the imaging core of the Vision Core Grant to the University of Washington, P30EY01730 (PI: Reh).
References
- Eiraku, M., Takata, N., Ishibashi, H., Kawada, M., Sakakura, E., Okuda, S., Sekiguchi, K., Adachi, T. and Sasai, Y. (2011). Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472(7341): 51-56.
- Eiraku, M. and Sasai, Y. (2012). Mouse embryonic stem cell culture for generation of three-dimensional retinal and cortical tissues. Nat Protoc 7(1): 69-79.
- Kamiya, D., Banno, S., Sasai, N., Ohgushi, M., Inomata, H., Watanabe, K., Kawada, M., Yakura, R., Kiyonari, H., Nakao, K., Jakt, L. M., Nishikawa, S. and Sasai, Y. (2011). Intrinsic transition of embryonic stem-cell differentiation into neural progenitors. Nature 470(7335): 503-509.
- La Torre, A., Hoshino, A., Cavanaugh, C., Ware, C. B. and Reh, T. A. (2015). The GIPC1-Akt1 pathway is required for the specification of the eye field in mouse embryonic stem cells. Stem Cells 33(9): 2674-2685.
- Nakano, T., Ando, S., Takata, N., Kawada, M., Muguruma, K., Sekiguchi, K., Saito, K., Yonemura, S., Eiraku, M. and Sasai, Y. (2012). Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 10(6): 771-785.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
La Torre, A. (2016). Retinal Differentiation of Mouse Embryonic Stem Cells. Bio-protocol 6(13): e1851. DOI: 10.21769/BioProtoc.1851.
Category
Stem Cell > Embryonic stem cell > Maintenance and differentiation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









