- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Root-knot Nematode Penetration and Sclareol Nematicidal Activity Assays
Published: Vol 6, Iss 12, Jun 20, 2016 DOI: 10.21769/BioProtoc.1848 Views: 11183
Reviewed by: Zhaohui LiuKrzysztof WieczorekAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Preparation of Nippostrongylus brasiliensis Larvae for the Study of Host Skin Response
Tiffany Bouchery [...] Nicola L. Harris
Dec 20, 2020 2912 Views

Quantification of Bacterial Loads in Caenorhabditis elegans
Alyssa C. Walker [...] Daniel M. Czyz
Jan 20, 2022 4241 Views

Botrytis cinerea in vivo Inoculation Assays for Early-, Middle- and Late-stage Strawberries
Piao Yang [...] Ye Xia
Oct 20, 2023 2776 Views
Abstract
Plant parasitic nematodes parasitize roots and/or stems of various plants thereby inhibiting absorption of nutrients and moisture. In particular, root-knot nematodes (RKN) are a group of the most devastating pests. Various techniques, such as soil sterilization, cultivation of resistant crops, and chemical application, have been developed to control damage caused by RKN. Among these techniques, diminish by chemicals that induce or activate host defense to RKN is an attractive method because of its potential to reduce the environmental burden caused by crop protection. Sclareol, a diterpene, was identified as a chemical that induces resistance to RKN (Fujimoto et al., 2015). Here we provide a protocol for assessing the impact of sclareol on the penetration of RKNs into tomato and Arabidopsis roots and the direct nematicidal impact of the chemical on nematodes. This protocol can be used for other nematode resistance-inducing chemicals.
Keywords: Root-knot nematodeMaterials and Reagents
- 220 ml bottom opened plastic pot
- Glass petri dish, 9 cm in diameter
Note: not necessary sterilized - Sterilized 50 ml glass culture bottle with cap
- 1 ml glass pipette
Note: Polypropylene pipette tips tend to retain Root-knot nematode (RKN), hence use of such tips should be avoided. - Sterilized 24-well tissue culture plate (e.g., IWAKI, catalog number: 3820-024 )
- RKN Meloidogyne incognita (NIAS Genebank, MAFF number: 108258 )
Note: We obtained a strain 108258 of RKN from the MAFF gene bank of the National Institute of Agrobiological Sciences of Japan (http://www.gene.affrc.go.jp/about_en.php). This strain can survive and propagate on plants carrying Mi-genes for RKN resistance (resistance breaking strain). - Tomato (Solanum lycopersicum), cultivar Momotaro
Note: The cultivar Momotaro carries Mi-genes for RKN resistance. Seeds of tomato cultivars possessing Mi-genes (e.g., Momotaro) can be obtained from a seed company (Takii & Co. Ltd., Kyoto, Japan). - Arabidopsis thaliana (Columbia)
- Water (Milli-Q grade)
- Sodium hypochlorite solution (Nacalai Tesque, catalog number: 31518-35 )
- Ethanol (99.5%) (Nacalai Tesque, catalog number: 09666-85 )
- Sea sand with an average size of 0.3 mm
Note: Shift and wash sea sand to remove the pebbles and the salt with a large amount of tap water in a metal tub and autoclave the washed sand. - Liquid fertilizer (Hyponex)
- Methanol (99.8%) (Nacalai Tesque, catalog number: 21915-93 )
- Acid fuchsin (e.g., Sigma-Aldrich, catalog number: F8129-25G , or Wako, catalog number: 061-01332 )
- Lactic acid (85~92%) (Nacalai Tesque, catalog number: 20006-75 )
- Glycerol (99%) (Nacalai Tesque, catalog number: 17018-83 )
- Sclareol (Sigma-Aldrich, catalog number: 357995-1G )
- Sodium hydroxide (Nacalai Tesque, catalog number: 31511-05 )
- Acetic acid (99%) (Nacalai Tesque, catalog number: 00211-95 )
- Acid fuchsin solution (see Recipes)
- 100 mM sclareol solution (see Recipes)
Equipment
- Greenhouse or chamber for plant growth
- Stainless tweezer
- Incubator (e.g., Sibata, model: SMU-0541 )
- Light microscope (e.g., OLYMPUS, model: BX53M )
- Refrigerator (e.g., PANASONIC, model: MPR-162DCN )
- Stainless steel tray (9.2 cm in height x 18 cm depth x 32 cm width)
- Scissors (e.g., V. Mueller, catalog number: 25601-164 )
- Water bath (e.g., Taitec, model: SH-10N )
Note: You can also use a commercially available microwave oven for cooking. - Microwave oven (e.g., HITACHI, model: MRO-NT5 )
- Stereoscopic microscope (e.g., Nikon, model: SMZ-U )
Procedure
- Propagation of RKN in tomato plants
- Suspend RKNs, which consist of a clonal population of RKN established from a single female, in water to a final concentration of at least 500 RKNs/ml.
Note: We use stock cultured RKNs obtained from field. - Inoculate 1 ml of RKNs suspension to 4-week-old tomato plants grown in a pot containing soil by injecting the solution on the soil surface surrounding the roots of each plant (Figure 1).

Figure 1. Inoculation of RKNs suspension on the soil surface using a 1 ml glass pipette - Grow the plants in a greenhouse maintained at 23-27 °C under natural sunlight for approximately 2 months.
- Carefully pull the roots out of the pots and wash them in tap water to remove the soil from the roots.
- Pick up the egg masses with tweezers from the roots.
- Suspend the collected egg masses in a small volume of water in a petri dish and incubate at 25 °C in the dark (Figure 2).

Figure 2. Petri dishes containing collected RKN egg-masses - After most juveniles hatched (about 10-14 days), vacuum the incubated suspension of nematodes using a glass pipette and release on a glass bottle (Figure 3).

Figure 3. Transfer of hatched RKN juveniles to a glass bottle - Suspend the collected RKNs in 5-10 ml water.
- Count the numbers of RKNs in the suspension under a light microscope (Figure 4).

Figure 4. Suspension of RKNs under a light microscope prior to the juveniles counting. Scale bar, 5 mm. - Prepare aliquots with an adequate concentration of RKNs in water.
Note: We adjust 500 RKNs/ml. You can store RKN suspensions without loss of activity in a refrigerator maintained at 10 °C for 2-3 weeks until use. - Use appropriate aliquots for each analysis.
- Suspend RKNs, which consist of a clonal population of RKN established from a single female, in water to a final concentration of at least 500 RKNs/ml.
- Plant growth
- Tomato plants
- Sterilize seeds for 3 min in 1.0% sodium hypochlorite solution.
- Wash the sterilized seeds in 70% ethanol.
- Wash the sterilized seeds three times in sterilized distilled water.
- Germinate the seeds on the sterilized wet paper at 23-27 °C with a photoperiod of 16 h of light and 8 h of dark.
- Transfer 7- to 10-day-old seedlings to autoclaved sand packed in 220 ml plastic pot and grow in a chamber maintained at 23-27 °C with a photoperiod of 16 h of light and 8 h of dark. Apply a liquid fertilizer diluted 3,000x with water to the pots every 3 days.
- Use tomato seedlings with two fully developed true leaves for each assay (Figure 5).

Figure 5. Tomato plant grown in soil - Arabidopsis plants
- Sterilized seeds for 3 min in 1.0% sodium hypochlorite solution.
- Wash the sterilized seeds in 70% ethanol.
- Wash the sterilized seeds three times in sterilized distilled water.
- Germinate the seeds on the sterilized 1.5% agar plate at 22 °C with a photoperiod of 16 h of light and 8 h of dark.
- Transfer 3-week-old seedlings to autoclaved sand packed in 220 ml plastic pot and grow in a chamber maintained at 22 °C with a photoperiod of 16 h of light and 8 h of dark.
- Apply a liquid fertilizer diluted 3,000x with water to the pots every 5 to 7 days.
- Use 10-week-old plants for each assay (Figure 6).
Note: We noted that Arabidopsis planted in sand grew slower than in soil. Thus, the size of 10-week-old plants grown in sand are almost the same as that of 6-week-old plants grown in soils.
Figure 6. Arabidopsis plants grown in vermiculite
- Sterilized seeds for 3 min in 1.0% sodium hypochlorite solution.
- Chemical treatments and RKN penetration assay
- Use 10 plants for each treatment.
- Place five plants per tray.
- Pour 2,000 ml of a solution containing adequate concentrations of sclareol or 0.1% methanol alone in the tray, as plant roots are submerged in the solution (Figure 7).
Note: We present here a protocol for sclareol, however, it can be also used for other chemicals. We used four concentrations; 50, 80, 100 and 200 μM.
Figure 7. The pots and tray used in our protocol - After submerging for 48 h, inoculate second-stage juveniles of RKNs to plants by injecting 1 ml of the RKN suspension (200 RKNs for tomato and 500 RKNs for Arabidopsis) on the sand surrounding the roots with 1 ml glass pipette.
Note: Because the efficiency of penetration of RKN to Arabidopsis roots is lower than that to tomato roots, we always use higher number of RKNs for Arabidopsis. - Grow the inoculated plants in a chamber maintained at 25 °C for tomato and 22 °C for Arabidopsis with a photoperiod of 16 h of light and 8 h of dark.
- Use 10 plants for each treatment.
- Staining with acid fuchsin
Note: Acid fuchsin is a dye that stains nematodes. The procedure described below is a modified version of the staining method reported by McBeth et al. (1941).- Seven days after inoculation, pull the roots out of the pots and wash them with tap water to remove the sea sand.
- Separate the roots from plants using scissors and put the roots in a 50 ml glass culture bottle.
- To decolorize the roots, pour 40 ml 1.0% sodium hypochlorite in the culture bottle and gently invert the bottle 10 or more times.
- Incubate for 5 min.
- After discarding the sodium hypochlorite, wash the roots with a large amount of tap water.
Note: Because sodium hypochlorite blocks the acid fuchsin’s staining effect, sodium hypochlorite should be completely removed at this step. - Pour 30 ml water in the culture bottle and then add 1 ml acid fuchsin. Mix well by inverting the bottle several times.
Note: The acid fuchsin solution in the bottle turns pale red. If the solution turns pale orange, it means that sodium hypochlorite still remains in the solution. - Boil the culture bottle containing the roots in the acid fuchsin solution in a preheated water bath at 100 °C.
Note: You can also use a microwave oven instead of water bath. In this case, the microwave oven should be stopped when the solution in the bottle starts to bubble. - Cool down the boiled roots and solution to room temperature.
- Discard the acid fuchsin solution and wash the roots with tap water to remove excess of acid fuchsin solution.
- Add 30 ml of a mixture of lactic acid and glycerol (1:1, v/v).
- Boil the bottle according to the step D7.
- Cool down the bottle to room temperature.
- After washing the roots with tap water, count the number of stained RKNs in the roots under a stereoscopic microscope. A representative example of stained RKNs is shown in Figure 8 and a representative result of sclareol-treated or non-treated plants for the penetration rate is shown in Figure 9.
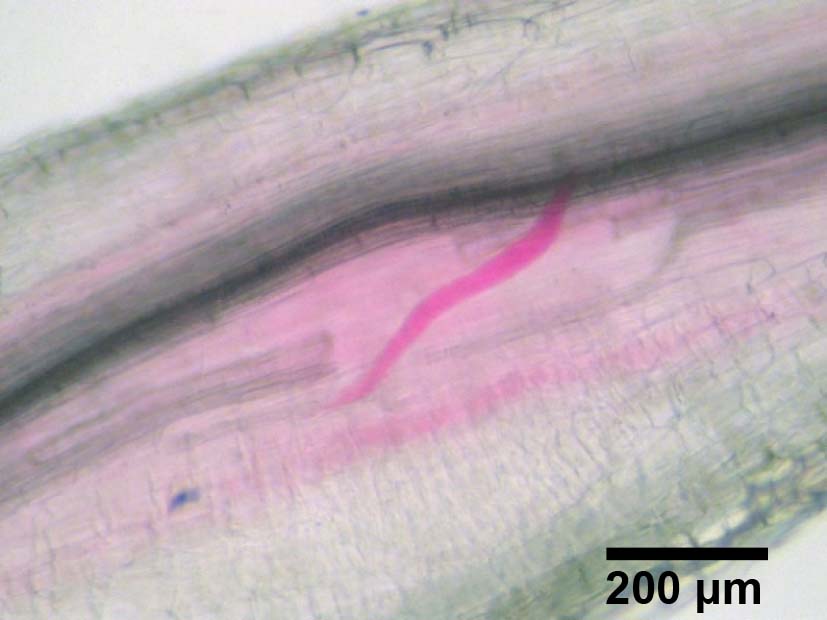
Figure 8. RKN in tomato roots stained with acid fuchsin. Scale bar, 200 μm.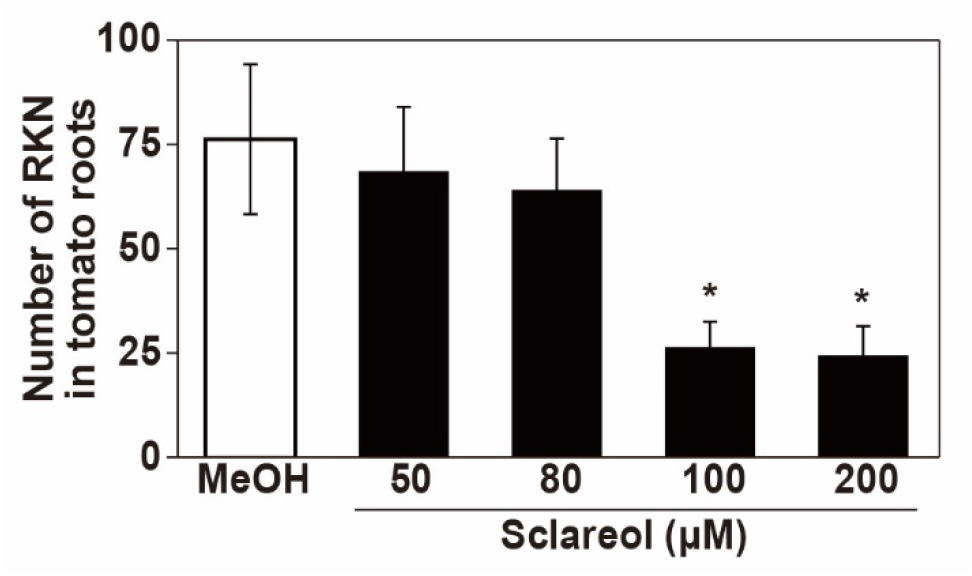
Figure 9. Effect of sclareol on the penetration of RKNs into tomato roots. Values are the mean ± SD from two independent experiments, each performed in 10 replicates. Asterisks denote statistically significant differences compared with the 0.1% methanol control (Tukey’s HSD test; P < 0.05).
- Seven days after inoculation, pull the roots out of the pots and wash them with tap water to remove the sea sand.
- Nematicidal activity assay
Note: The procedure described below is a modified version of the assay reported by Chen and Dickson (2000).- Dilute RKN suspension to a concentration of 100 RKNs per ml with water.
- Add 1 ml RKN suspension to each well of 24-well tissue culture plates.
- Add 1 ml solution containing adequate concentration of sclareol or 0.1% methanol to each well.
- Incubate the plates in the dark at 25 °C.
- Count the numbers of motile and non-motile RKNs 1, 3, 6, 12, 24 and 48 h after the chemical application under a light microscope.
- To check motility of RKNs, add a few drops of 1 M sodium hydroxide (NaOH) to each well.
Note: Sodium hydroxide can stimulate movement of RKN. If RKN moves by the addition of NaOH, we count it as living RKN. - Calculate the ratio of living RKN to dead RKN (Figure 10).
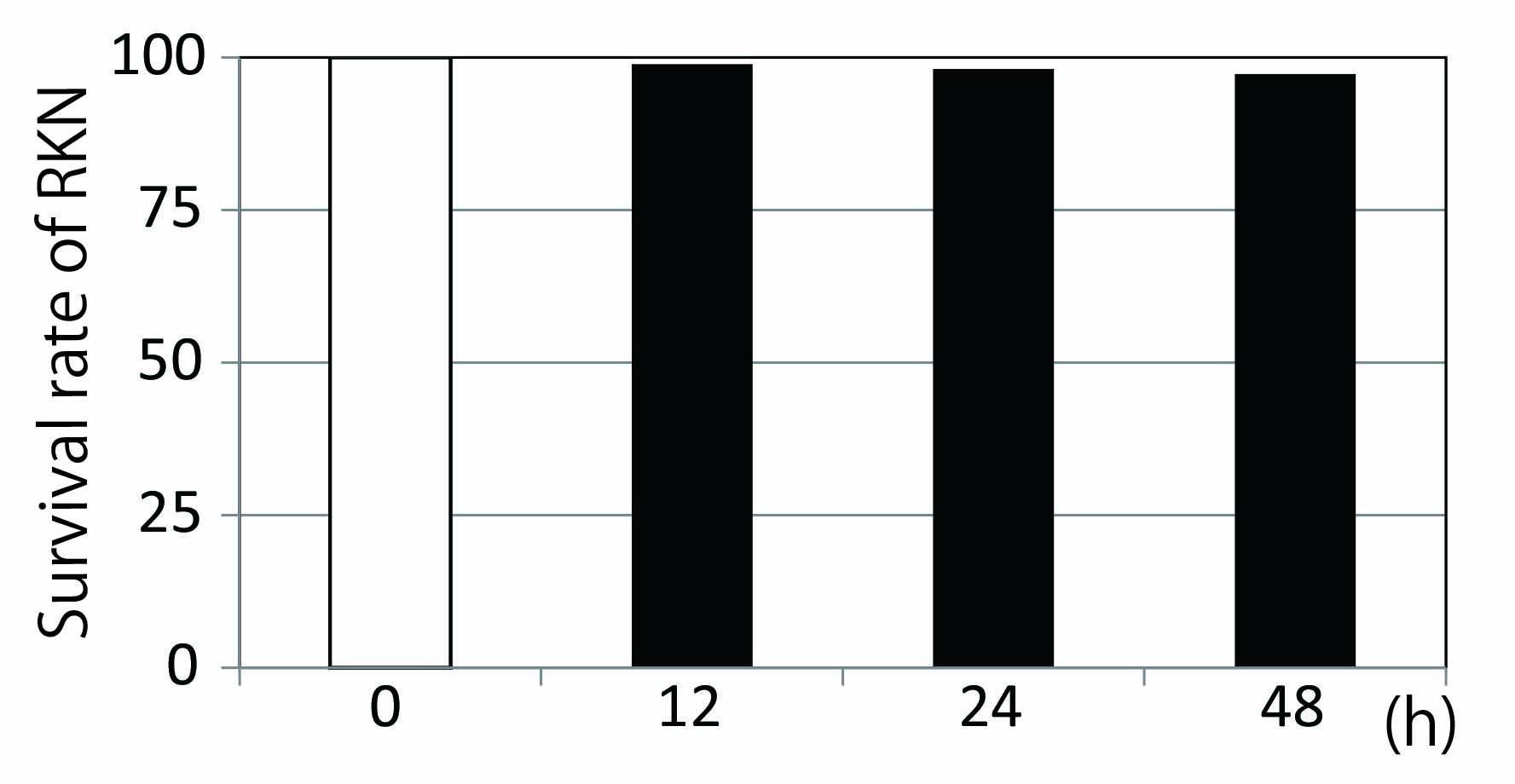
Figure 10. Nematicidal activity of 100 μM sclareol. Approximately 100 RKNs in 1 ml of nematode suspension were added to 1 ml of 200 μM sclareol solution or control solution (0.2% methanol) in 24-well tissue culture plates and incubated in the dark at 25 °C. At 1, 3, 6, 12, 24 and 48 h after the onset of incubation, the numbers of motile and non-motile RKNs were counted (data for 1, 3 and 6 h are not shown).
- Dilute RKN suspension to a concentration of 100 RKNs per ml with water.
Recipes
- Acid fuchsin solution (for 1,000 ml)
Dissolve 2.5 g acid fuchsin in 500 ml water and add 250 ml acetic acid to the solution
Adjusted with water to the final volume of 1,000 ml
Stored at room temperature in the dark - 100 mM sclareol solution
Dissolve sclareol in methanol to a concentration of 100 mM
You can use the solution as a stock solution
Store the stock solution at -20 °C in the dark
Dilute the stock solution to each concentration with water
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, the Japanese Program for the Promotion of Basic and Applied Research for Innovation in Bio-oriented Industry (BRAIN), Japan, and a grant from Cross-ministerial Strategic Innovation Promotion Program, Japan.
References
- Chen, S. Y. and Dickson, D. W. (2000). A technique for determining live second-stage juveniles of Heterodera glycines. J Nematol 32:117-121.
- Fujimoto, T., Mizukubo, T., Abe, H. and Seo, S. (2015). Sclareol induces plant resistance to root-knot nematode partially through ethylene-dependent enhancement of lignin accumulation. Mol Plant Microbe Interact 28: 398-407.
- McBeth, C. W., Taylor, A. L. and Smith, A. L. (1941). Note on staining nematodes in root tissues. Proc Helminthol Soc Wash 16: 3-6.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Fujimoto, T., Mizukubo, T., Abe, H. and Seo, S. (2016). Root-knot Nematode Penetration and Sclareol Nematicidal Activity Assays. Bio-protocol 6(12): e1848. DOI: 10.21769/BioProtoc.1848.
Category
Plant Science > Plant immunity > Disease bioassay
Microbiology > Microbe-host interactions > Nematode
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









