- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Extraction and Quantification of Sphingosine 1-Phosphate (S1P)
Published: Vol 6, Iss 10, May 20, 2016 DOI: 10.21769/BioProtoc.1817 Views: 10062
Reviewed by: Ivan ZanoniYang FuAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Flow-cytometric Detection of Low-level Reactive Oxygen Species in Cell Lines and Primary Immune Cells
Kevin Bode [...] Heiko Weyd
Sep 5, 2020 7243 Views

Sample Preparation and Integrative Data Analysis of a Droplet-based Single-Cell ATAC-sequencing Using Murine Thymic Epithelial Cells
Tatsuya Ishikawa [...] Taishin Akiyama
Jan 5, 2023 2415 Views

Proliferation Assay Using Cryopreserved Porcine Peripheral Mononuclear Cells Stimulated With Concanavalin A and Analyzed With FCS ExpressTM 7.18 Software
Marlene Bravo-Parra [...] Luis G. Giménez-Lirola
Jun 5, 2025 2619 Views
Abstract
Sphingosine 1-phosphate (S1P) is a lipid metabolite and signaling molecule involved in many different physiological processes including lymphocyte circulation, T cell differentiation, antigen presentation, and maintenance of the vascular endothelial barrier. S1P is a ligand of five different G protein-coupled cell surface receptors designated S1P1-5. It has also been described as an intracellular second messenger. Quantification of S1P in biological samples is therefore an important task to decipher its signaling capabilities in vivo under physiological and pathophysiological conditions in different body fluids and organs. In this protocol, quantification of S1P is performed by liquid chromatography coupled to triple-quadrupole mass spectrometry (LC-MS/MS).
Keywords: Liquid chromatographyMaterials and Reagents
- Chloroform (CHCl3) (HPLC-Grade) (Carl Roth GmbH + Co., catalog number: 7331.1 )
- Methanol (MeOH) (HPLC-Grade) (VWR International, catalog number: 20864.320 )
- Formic acid (Carl Roth, catalog number: 4742.1 )
- Sphingosine 1-phosphate (S1P) (Sigma-Aldrich, catalog number: S9666 )
- C17-S1P (Avanti Polar Lipids, catalog number: 860641P )
- Hydrochloric acid (HCl) (37%) (Carl Roth GmbH + Co., catalog number: 9277.1 )
- Sodium chloride (NaCl) (Carl Roth GmbH + Co., catalog number: 3957.3 )
- Potassium chloride (KCl) (Carl Roth GmbH + Co., catalog number: 6781.3 )
- di-Sodium hydrogen phosphate dehydrate (Na2HPO4.2H2O) (Carl Roth GmbH + Co., catalog number: 4984.2 )
- Potassium dihydrogen phosphate (KH2PO4) (Carl Roth GmbH + Co., catalog number: 3904.1 )
- Phosphate-buffered saline (see Recipes)
Equipment
- S1P extraction
- VX-2500 vortexer (VWR International, catalog number: 58816-116 )
- Pyrex® glass centrifuge vials (VWR International, catalog number: 734-4240 )
- RVC 2-25 CD plus vacuum concentrator (Christ)
- Autosampler vials (VWR International, catalog number: 548-0029 )
Note: It is also named “Short thread vials, ND9” on VWR International website. - Inserts for autosampler vials (VWR International, catalog number: 548-3006 )
Note: It is also named “Screw neck vials, ND10” on VWR International website. - Screw caps for autosampler vials (VWR International, catalog number: 548-0382 )
- VX-2500 vortexer (VWR International, catalog number: 58816-116 )
- LC-MS/MS
- Binary pump 1100 series HPLC system (Hewlett Packard/Agilent)
- 2 x 60 mm MultoHigh C18-RP column, 3 µm particle size (CS Chromatographie-Service GmbH, catalog number: 536201 )
- 2000 QTrap LC/MS/MS system (AB Sciex)
- Binary pump 1100 series HPLC system (Hewlett Packard/Agilent)
Software
- Analyst 1.6.2 (AB Sciex)
Procedure
- S1P extraction protocol
Note: All following steps are performed at room temperature if not stated otherwise.- Transfer the sample (plasma, medium, cell suspension) into a glass centrifuge vial and adjust the volume to 1 ml with PBS. Samples were prepared as follows:
- 50-200 µl plasma was taken from heparinized blood.
- 1 ml medium was directly taken from cell culture.
- Cells were trypsinized, washed once in PBS and taken up in 1 ml PBS.
Note: All samples can be processed directly or stored at -20 °C to -80 °C until use.
- 50-200 µl plasma was taken from heparinized blood.
- Add 10 µl of the internal standard (10 μM C17- S1P in MeOH).
- Add 300 µl of 18.5% HCl.
- Add 1 ml MeOH and 2 ml CHCl3.
- Vortex for 10 min at maximum speed.
- Centrifuge the sample for 3 min at 1,900 x g.
- Transfer the lower CHCl3 phase into a new glass centrifuge vial by directly placing the pipet into the lower phase (see Figure 1).
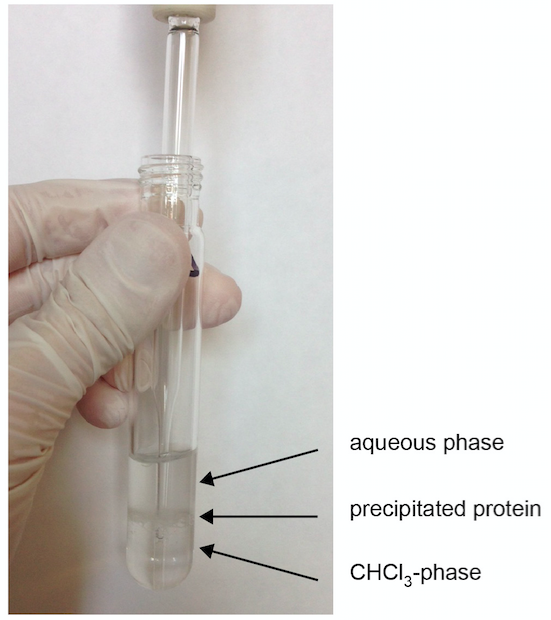
Figure 1. Formation of phases after centrifugation. As an example, S1P extraction from a plasma sample is shown in step A7. The CHCl3-phase is extracted by directly pipetting through the upper aqueous phase. - Add 2 ml of CHCl3 to the remaining aqueous phase and repeat vortexing and centrifugation.
- Add this CHCl3-phase to the transferred CHCl3-phase of step A7.
- Vacuum-dry the CHCl3 in the vacuum rotator at 60 °C for 45 min. Alternatively, the samples can be dried under nitrogen gas flow.
- Resuspend the sample in 100 µl MeOH:CHCl3 (4:1, vol/vol).
- Vortex the sample for 1 min at maximum speed.
- Transfer the sample into an autosampler vial and store it at -20 °C.
- Transfer the sample (plasma, medium, cell suspension) into a glass centrifuge vial and adjust the volume to 1 ml with PBS. Samples were prepared as follows:
- MS protocol
- HPLC-program
- Solution A: ddH2O containing 1% formic acid.
- Solution B: MeOH.
- Use a flow-rate of 0.3 ml/min.
- Equilibrate the column for 5 min with 90% Solution A and 10% solution B.
- From 0-0.5 min: Change to 100% solution B.
- From 0.5-15 min: Hold 100% solution B.
- From 15.1-20 min: Re-equilibrate with 90% solution A and 10% solution B.
- Solution A: ddH2O containing 1% formic acid.
- 10 µl of the sample is applied onto the column 1 min after starting the HPLC program.
- The column is kept at 35 °C during the whole procedure.
- The spectrum is acquired with an electrospray ionization (ESI) ion source in the positive mode and following settings:
- Ion spray voltage: 4,500
- Ion source heater temperature: 450 °C
- Collision gas setting: Medium
- Ion source gas 1: 30 psi
- Ion source gas 2: 60 psi
- Curtain gas: 45 psi
- Ion spray voltage: 4,500
- For acquisition the multiple reaction monitoring (MRM) mode and the Analyst 1.6.2 software is used. S1P is analyzed with the mass transition 380 m/z -> 264 m/z, and the internal standard C17- S1P with the mass transition 366 m/z -> 250 m/z.
- For quantitative analysis a standard curve with S1P amounts of 1 pmol to 100 pmol and 10 pmol C17- S1P as the internal standard is generated.
- S1P concentrations are calculated using Analyst 1.6.2 software.
- HPLC-program
Representative data
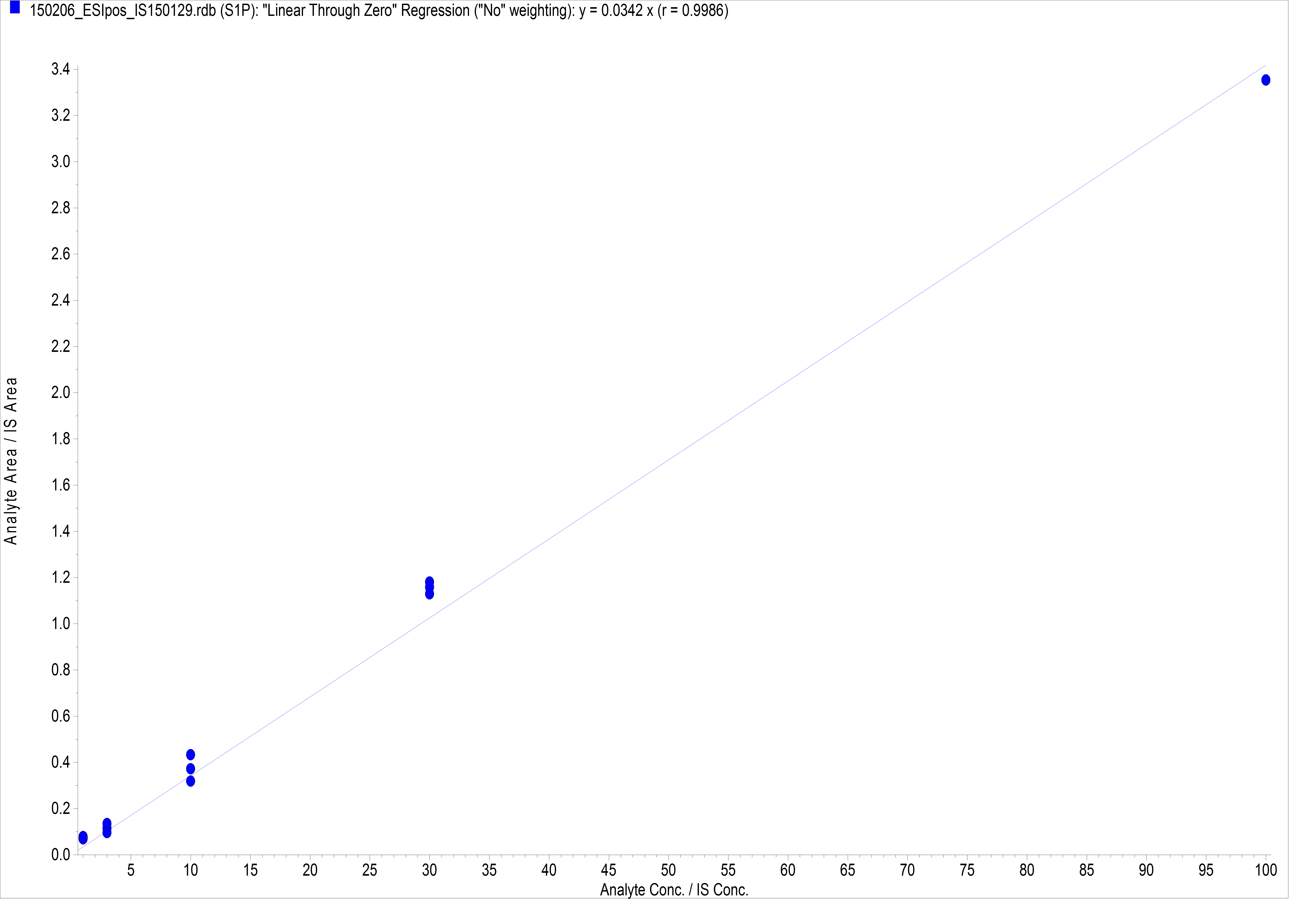
Figure 2. Example of S1P standard curve. For the generation of the standard curve, each concentration is measured three times. For this curve the following S1P amounts were used: 1 pmol, 3 pmol, 10 pmol, 30 pmol, 100 pmol.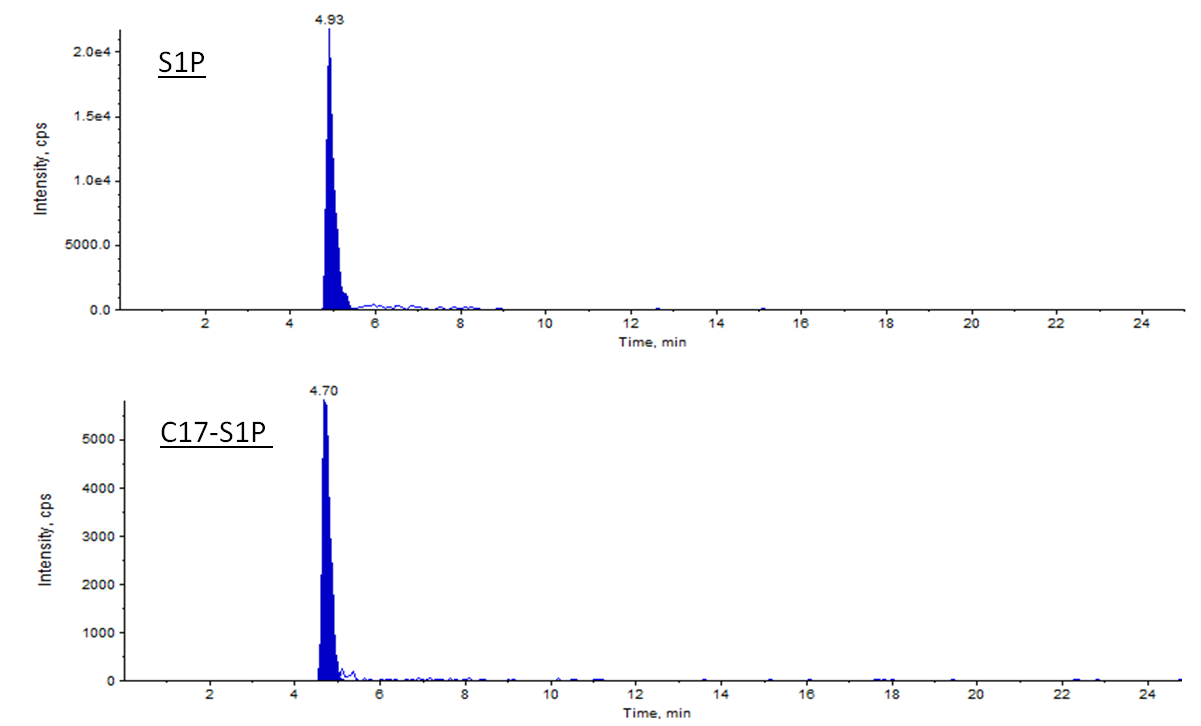
Figure 3. Example of S1P spectrum acquired with ESI ion source in positive mode. Representative signals of S1P and its respective internal standard C17-S1P in 10 µl of the 10 µM standard are plotted. The total amounts of S1P and C17-S1P are 100 pmol and 10 pmol, respectively. Retention times of S1P and C17-S1P are slightly different (4.93 min vs. 4.70 min).
Notes
- If S1P needs to be measured in tissue samples, homogenize up to 50 mg tissue (might need to be adjusted depending on the lipid concentration of the tissue) together with 10 µl of the internal standard (10 µM C17- S1P in MeOH) in 1 ml PBS. Transfer the homogenate into a glass centrifuge vial and proceed with step A3 of the S1P extraction protocol.
- The extraction efficiency is close to 100% for S1P (Andréani and Gräler, 2006).
Recipes
- Phosphate-buffered saline
140 mM NaCl
2.7 mM KCl
10 mM Na2HPO4.2H2O
1.8mM KH2PO4
Adjust pH to 7.4
Acknowledgments
The extraction and measurement method is adapted from Bode and Gräler (2012).
References
- Andréani, P. and Gräler, M. H. (2006). Comparative quantification of sphingolipids and analogs in biological samples by high-performance liquid chromatography after chloroform extraction. Anal Biochem 358(2): 239-246.
- Bode, C. and Graler, M. H. (2012). Quantification of sphingosine-1-phosphate and related sphingolipids by liquid chromatography coupled to tandem mass spectrometry. Methods Mol Biol 874: 33-44.
- Sattler, K., Graler, M., Keul, P., Weske, S., Reimann, C. M., Jindrova, H., Kleinbongard, P., Sabbadini, R., Brocker-Preuss, M., Erbel, R., Heusch, G. and Levkau, B. (2015). Defects of high-density lipoproteins in coronary artery disease caused by low sphingosine-1-phosphate content: Correction by sphingosine-1-phosphate-loading. J Am Coll Cardiol 66(13): 1470-1485.
- Vettorazzi, S., Bode, C., Dejager, L., Frappart, L., Shelest, E., Klassen, C., Tasdogan, A., Reichardt, H. M., Libert, C., Schneider, M., Weih, F., Henriette Uhlenhaut, N., David, J. P., Graler, M., Kleiman, A. and Tuckermann, J. P. (2015). Glucocorticoids limit acute lung inflammation in concert with inflammatory stimuli by induction of SphK1. Nat Commun 6: 7796.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Reimann, C. and Gräler, M. H. (2016). Extraction and Quantification of Sphingosine 1-Phosphate (S1P). Bio-protocol 6(10): e1817. DOI: 10.21769/BioProtoc.1817.
Category
Immunology > Immune cell function > General
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











