- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
3D Gel Invasion Assay of Gastric Cancer Cells with Fibroblasts
Published: Vol 6, Iss 9, May 5, 2016 DOI: 10.21769/BioProtoc.1798 Views: 13015
Reviewed by: HongLok LungAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Calvarial Bone Implantation and in vivo Imaging of Tumor Cells in Mice
Kyoko Hashimoto [...] Mitsuru Futakuchi
Feb 5, 2019 8181 Views

Bone-in-culture Array to Model Bone Metastasis in ex vivo Condition
Hai Wang and Xiang H.-F. Zhang
Jan 20, 2020 4975 Views

Cell-derived Matrix Assays to Assess Extracellular Matrix Architecture and Track Cell Movement
Kendelle J. Murphy [...] David Herrmann
Dec 20, 2022 3370 Views
Abstract
Cancer tissue is composed of cancer cells and a large number of stromal cells including fibroblasts. In order to understand the relationship between fibroblasts and cancer cells during invasion of the stroma, 3D gel invasion assay is useful. Most tumors are associated with a biologically active type of fibroblasts known as cancer-associated fibroblasts (CAFs), which promote the invasion of cancer cells. Here, we describe the method of imaging the invasion by fluorescently labeled CAFs and gastric cancer cells in gels containing extracellular matrix. For two-color fluorescence labeling of living cells, long-chain dialkylcarbocyanines, DiO and DiI were used. This method is also applicable for studying invasion by other stromal cells and cancer cells, and for evaluation of drugs targeting cancer stromal cells.
Keywords: CAFMaterials and Reagents
- Collagen-coated dish (Sanyo, IWAKI, catalog number: 4010-010 )
- Transparent PET membrane 24 well 3.0 μm pore size (Corning, Falcon®, catalog number: 353096 )
- 24 well plate for use with cell culture inserts (Corning, Falcon®, catalog number: 353504 )
- Razor blades (Esbjerg, Feather, catalog number: FA-10 )
- Microslide glass (Matsunami Glass Ind, catalog number: TF0215M )
- Micro cover glass, 24 x 24 mm (Thickness NO.1: 0.12-0.17 mm) (Matsunami Glass Ind)
- Gastric cancer cells (44As3) (Yanagihara K et al., 2005)
Note: It’s established from gastric cancer patient. - Fibroblasts (CAF) (Fuyuhiro Y et al., 2011)
Note: It’s isolated from surgical materials of gastric cancer patients. - Type I-collagen (Nitta Gelatin Inc., Cellmatrix Type I-P)
- Matrigel matrix (Corning, catalog number: 356234 )
- 3, 3’-dioctadecycloxacarbo-cyanine perchlorate (DiO) (Thermo Fisher Scientific, Molecular Probes™, catalog number: D-275 )
- 1, 1’-dioctadecyl-3, 3, 3’, 3’-tetramethyllindo-carbocyanine perchlorate (DiI) (Thermo Fisher Scientific, Molecular Probes™, catalog number: D-282 )
- Trypsin-EDTA solution (Sigma-Aldrich, catalog number: T3924 )
- Penicillin-Streptomycin (Sigma-Aldrich, catalog number: P4333 )
- Dulbecco’s modified Eagle’s medium (Sigma-Aldrich, catalog number: D6046 )
- RPMI-1640 medium (Sigma-Aldrich, catalog number: R8758 )
- 10x Dulbecco’s modified Eagle’s medium (Sigma-Aldrich, catalog number: D2429 )
- 10x RPMI-1640 medium (Sigma-Aldrich, catalog number: R1145-500ML )
- Fetal bovine serum (heat inactivated) (Sigma life science, catalog number: 172012-500ML , batch: S13C490)
- Phosphate buffered saline (PBS) (Sigma-Aldrich, catalog number: P4417 )
- 4% Paraformaldehyde phosphate buffer (Wako Pure Chemical Industries, catalog number: 163-20145 )
- Polyvinyl alcohol mounting medium (Sigma-Aldrich, Fluka, catalog number: 10981 )
- Instant glue (Krazy Glue, catalog number: KG585 )
- NaHCO3 (Sigma-Aldrich, catalog number: S6014-500G )
- NaOH (Sigma-Aldrich, catalog number: S5881-500G )
- HEPES (Sigma-Aldrich, catalog number: H7006-25G )
- Gel (0.2 mg/ml type I-collagen and 2.5 mg/ml matrigel matrix) (see Recipes)
- Reconstitution buffer (see Recipes)
Equipment
- Tweezers (Electron Microscopy Sciences, Dumont, model: No.5 )
- 37 °C, 5% CO2 incubator (LabX, Sanyo, model: MCO-19AIC )
- Dissecting microscope (OLYMPUS CORPORATION, model: SZ61 )
- Light source (OLYMPUS CORPORATION, KL1600LED )
- Vibratome (DOSAKA EM, model: LinearSlicer PRO7 )
- Confocal laser scanning microscope (ZEISS, model: LSM 780 )
Procedure
- 44As3 cells and gastric CAFs were cultured in RPMI-1640 medium or DMEM, respectively supplemented with 10% FBS and Penicillin (100 unit/ml)-Streptomycin (0.1 mg/ml) at 37 °C in a humidified atmosphere containing 5% CO2. CAFs were maintained in collagen-coated dishes.
- 44As3 cells and CAFs were labeled with DiO or DiI, respectively as follows. Stock solutions of DiO and DiI were prepared in dimethylformamide at 2.5 mg/ml. DiO or DiI was added in the medium at final concentration of 6 μg/ml, and cells were incubated for 1 h in a CO2 incubator. Cells were then washed by replacing with fresh medium containing 10% FBS and backed into a CO2 incubator for 20 min, repeat this step two cycles.
- During labeling the cells, prepare the gel. Serum-free DMEM/RPMI-1640 (1:1) medium containing 0.2 mg/ml type I-collagen and 2.5 mg/ml matrigel matrix (refer to the below recipe) was laid onto the upper chamber of transwells (150 μl/ well), and solidify in a CO2 incubator at 37 °C for 30 min.
- 44As3 cells and CAFs were detached by trypsin-EDTA (trypsin 0.5 g/L, incubate 3 min) and mixed (1.5 x 104 cells each) in 200 μl of medium composed by 1:1 mixture of DMEM and RPMI-1640 supplemented with 0.2% FBS, and placed on gels. The lower compartment of the transwell was filled with 700 μl of the same medium with 10% FBS. Cells were incubated for 5-9 days with replacing the upper and lower medium every other day (Figure 1).
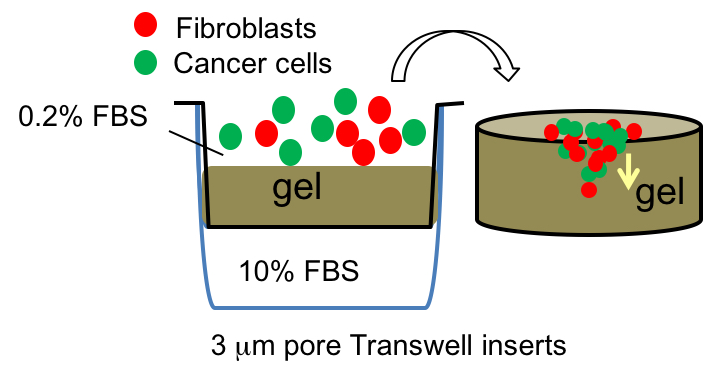
Figure 1. Cell-culture on the gel in transwell inserts. (Left). Mixture of cancer cells and fibroblasts, labeled with distinguishable fluorescence dyes were put on the gel, which was prepared in 3.0 μm pored transwell inserts. (Right). The cells invade into the gel according to serum gradient. - Take the gels out from transwell inserts by cutting the insert membrane along the whole circumference (Video 1). The gels were fixed in 4% paraformaldehyde for 1 h at room temperature, or overnight at 4 °C. If necessary, take photos of the whole gel as activated fibroblasts contract the gel (Figure 2A).
- The fixed gel was once rinsed with PBS. Periphery of the gel was excised by a razor blade under dissecting microscope (Figure 2B). The edge of cell area, which is usually visible by labeled fluorescence is excised. The gel was attached to the stage of vibratome slicer by instant glue (Figure 3A, B), and cut into 200 μm thick slices in PBS (Figure 3C-D, Video 2).
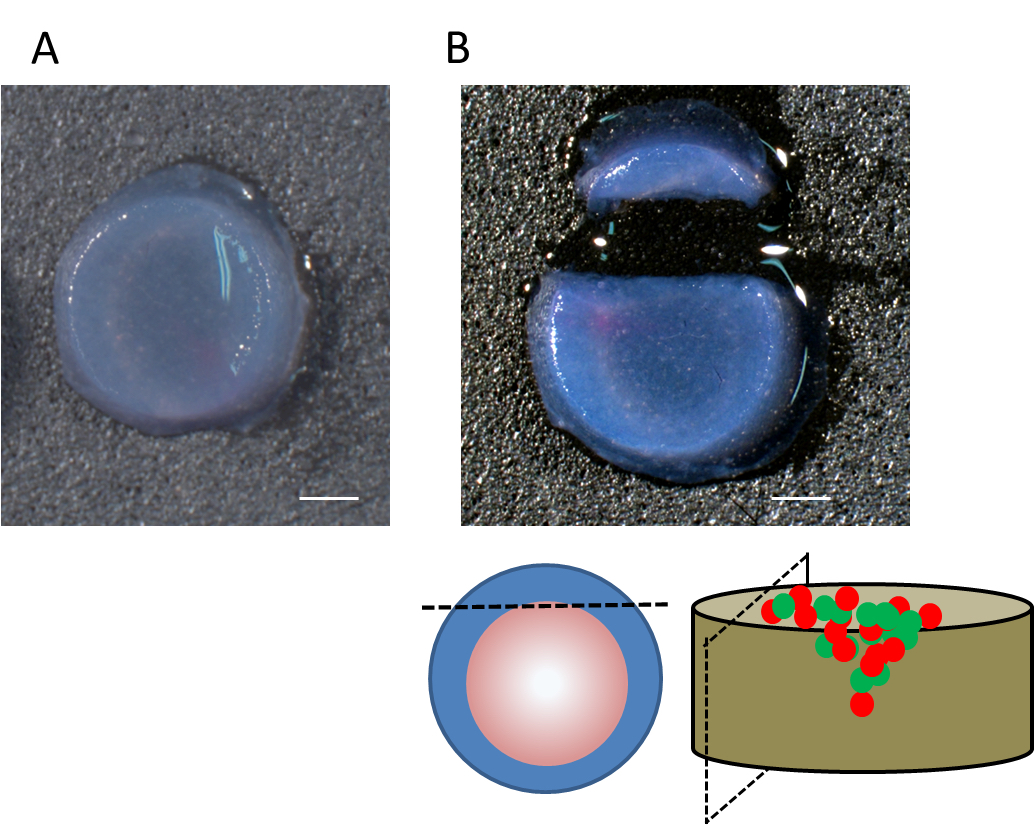
Figure 2. Appearance of the gel before setting on the vibratome. A. Appearance of the whole gel after taking out from the insert and fixation. B. After excision of peripheral part of the gel. The position of excision was shown at the bottom.
Figure 3. Slice of the gel by a vibratome. A, B. Attach the gel face a section down to the metal stage of a vibratome. A. Superior view, B. Lateral view of the gel. C. The stage is filled with PBS. The gel is sectioned by a razor blade in PBS. D. Direction of the sectioning was shown. - The slices were picked up from PBS, put on a glass slide, and fix the shape of the slices by straightening them with tweezers under dissecting microscope. Wipe excess PBS by a paper and mounted in polyvinyl alcohol mounting medium (Figure 4).

Figure 4. Appearance of sliced gels. A. Illustrations of sliced gels. B. Sliced gels were arranged on a slide glass. Bar = 1 mm - After solidified the mounting medium, the slices were visualized using a confocal microscope. The 3D-rendered images are obtained from the z-stack images (Figure 5).
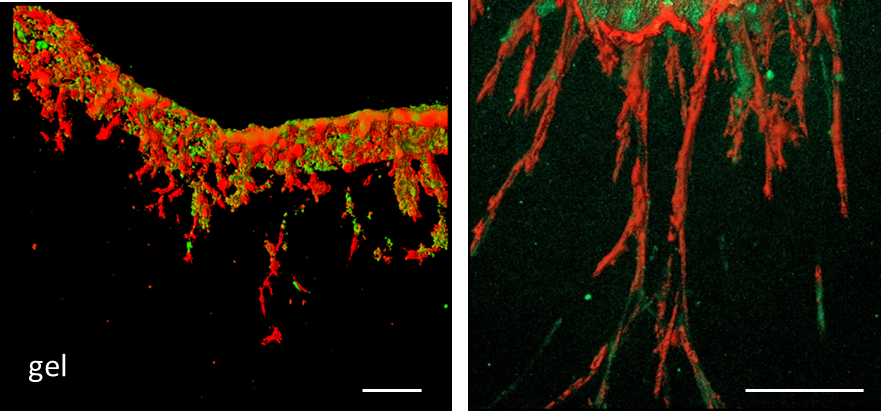
Figure 5. Representative confocal images of a gel section. Green: Cancer cells, Red: CAFs. Mixture of cancer (44As3) cells and CAFs protruded and invaded into the gel. Gels were fixed at day 7. Bar = 100 μm
Representative data
Notes
The optimum length of culture period should be determined empirically. Usually between 5-9 days. If solidified glue was attached to the sliced gel, it should be removed in PBS using tweezers under dissecting microscope before mounting.
Recipes
- Gel (0.2 mg/ml type I-collagen and 2.5 mg/ml matrigel matrix)
Mix type-I collagen gel and matrigel matrix (3:1). For example, if you make total 800 μl mixed gel, at first prepare 600 μl of type-I collagen by mixing 10x conc. culture medium (60 μl), reconstitution buffer (60 μl) and type-I collagen (480 μl) in a tube on ice. Add 200 μl of matrigel matrix and mix thoroughly. - Reconstitution buffer
2.2 g NaHCO3 in 100 ml of 0.05 N NaOH and 200 mM HEPES
Acknowledgments
This protocol was adapted from the previously published studies, Satoyoshi et al. (2015a; 2015b) and Tsuji et al. (2015). This work was supported by JSPS KAKENHI (Grant Nos. 25290042, 26640068) and Research Grant of the Princess Takamatsu Cancer Research Fund (No. 14-24620).
References
- Fuyuhiro, Y., Yashiro, M., Noda, S., Kashiwagi, S., Matsuoka, J., Doi, Y., Kato, Y., Hasegawa, T., Sawada, T. and Hirakawa, K. (2011). Upregulation of cancer-associated myofibroblasts by TGF-beta from scirrhous gastric carcinoma cells. Br J Cancer 105(7): 996-1001.
- Satoyoshi, R., Kuriyama, S., Aiba, N., Yashiro, M. and Tanaka, M. (2015a). Asporin activates coordinated invasion of scirrhous gastric cancer and cancer-associated fibroblasts. Oncogene 34(5): 650-660.
- Satoyoshi, R., Aiba, N., Yanagihara, K., Yashiro, M. and Tanaka, M. (2015b). Tks5 activation in mesothelial cells creates invasion front of peritoneal carcinomatosis. Oncogene 34(24): 3176-3187.
- Tsuji, T., Satoyoshi, R., Aiba, N., Kubo, T., Yanagihara, K., Maeda, D., Goto, A., Ishikawa, K., Yashiro, M. and Tanaka, M. (2015). Agr2 mediates paracrine effects on stromal fibroblasts that promote invasion by gastric signet-ring carcinoma cells. Cancer Res 75(2): 356-366.
- Yanagihara, K., Takigahira, M., Tanaka, H., Komatsu, T., Fukumoto, H., Koizumi, F., Nishio, K., Ochiya, T., Ino, Y. and Hirohashi, S. (2005). Development and biological analysis of peritoneal metastasis mouse models for human scirrhous stomach cancer. Cancer Sci 96(6): 323-332.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Tanaka, M. (2016). 3D Gel Invasion Assay of Gastric Cancer Cells with Fibroblasts. Bio-protocol 6(9): e1798. DOI: 10.21769/BioProtoc.1798.
Category
Cancer Biology > Invasion & metastasis > Tumor microenvironment
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










