- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Visualization of Intracellular Tyrosinase Activity in vitro
(*contributed equally to this work) Published: Vol 6, Iss 8, Apr 20, 2016 DOI: 10.21769/BioProtoc.1794 Views: 9805
Reviewed by: Ralph BottcherHsin-Yi ChangAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Fluorescence Polarization-Based High-Throughput Screening Assay for Inhibitors Targeting Cathepsin L
Keyu Guo [...] Shuyi Si
Jul 20, 2025 2282 Views

Intraepidermal Nerve Fiber Quantification of the Mouse Hind Paw Footpads: A Detailed and Simplified Protocol
Anastasia Yerushkin [...] Amir Dori
Dec 5, 2025 1247 Views

Detecting the Activation of Endogenous Small GTPases via Fluorescent Signals Utilizing a Split mNeonGreen: Small GTPase ActIvitY ANalyzing (SAIYAN) System
Miharu Maeda and Kota Saito
Jan 5, 2026 496 Views
Abstract
Melanocytes produce the melanin pigments in melanosomes and these organelles protect the skin against harmful ultraviolet rays. Tyrosinase is the key cuproenzyme which initiates the pigment synthesis using its substrate amino acid tyrosine or L-DOPA (L-3, 4-dihydroxyphenylalanine). Moreover, the activity of tyrosinase directly correlates to the cellular pigmentation. Defects in tyrosinase transport to melanosomes or mutations in the enzyme or reduced intracellular copper levels result in loss of tyrosinase activity in melanosomes, commonly observed in albinism. Here, we describe a method to detect the intracellular activity of tyrosinase in mouse melanocytes. This protocol will visualize the active tyrosinase present in the intracellular vesicles or organelles including melanosomes.
Keywords: TyrosinaseMaterials and Reagents
- Glass coverslips (diameter-12 mm, No.1) (Polar Industrial Corporation, Blue Star, catalog number: 12mm Circular )
Note: See Recipes for acid wash and sterilization. - Micro slides (L-75 mm x W-25 mm x h-1.35 mm) (Polar Industrial Corporation, Blue Star, catalog number: PIC-1 )
- Plastic tissue culture (6 well) plate (Corning, catalog number: 3506 ) and bottle-top vacuum filter (pore size 0.22 μm) (Corning, catalog number: 430015 )
- Melanocytes (Immortal wild type mouse melanocytes, melan-Ink4a-Arf-1 from C57BL/6J mice, referred to here as melan-Ink4a) [Resource: The Wellcome Trust Functional Genomics Cell Bank (Sviderskaya et al., 2010)]
- Copper(II) sulphate pentahydrate (CuSO4·5H2O) (Sigma-Aldrich, catalog number: C7631 )
- 3, 4-Dihydroxy-D-phenylalanine (D-DOPA) (Sigma-Aldrich, catalog number: D9378 )
- 3, 4-Dihydroxy-L-phenylalanine (L-DOPA) (Sigma-Aldrich, catalog number: D9628 )
- HCl (Sigma-Aldrich, catalog number: H1758 )
- HNO3 (Merck Millipore, catalog number: 101799 )
- KCl (Sigma-Aldrich, catalog number: P9541 )
- KH2PO4 (Merck Millipore, catalog number: 104873 )
- NaCl (Fisher Scientific, catalog number: BP358-1 )
- Na2HPO4·2H2O (Fisher Scientific, catalog number: S472-500 )
- Ethanol (70%) (Merck Millipore, catalog number: 818760 )
- Fetal bovine serum (Biowest, catalog number: S1810-500 )
- Formaldehyde (36.5-38% in H2O) solution (HCHO solution) (Sigma-Aldrich, catalog number: F8775 )
- Fluromount-G or mounting medium (SouthernBiotech, catalog number: 0100-01 )
- Matrigel Matrix (Corning Matrigel Growth Factor Reduced Basement Membrane Matrix, Phenol Red-Free) (Corning, catalog number: 356231 )
- Penicillin-Streptomycin (antibiotic) (Thermo Fisher Scientific, GibcoTM, catalog number: 15140-122 )
- RPMI-1640 media (Thermo Fisher Scientific, GibcoTM, catalog number: 31800-022 )
- L-Glutamine (Thermo Fisher Scientific, GibcoTM, catalog number: 25030-081 )
- 0.1% DOPA solution (see Recipes)
- 4% Formaldehyde solution (see Recipes)
- Growth media (see Recipes)
- 0.1% Matrigel matrix solution (see Recipes)
- 1x PBS (see Recipes)
Equipment
- CO2 incubator (maintained at 37 °C, 10% CO2) (Thermo Fisher Scientific, model: Forma Water Jacketed CO2 incubator )
- Forceps (sterilized by autoclave)
- Bright field microscope (Olympus Corporation, model: IX81 motorized inverted fluorescence microscope )
- Hot-air-oven (Eyela, catalog number: NDO-420W )
- Glass beaker (250 ml) (Borosil, catalog number: 1000D21 )
Procedure
- Prepare coverslips for the following treatments: 1x PBS and/or D-DOPA (negative control), L-DOPA and L-DOPA with copper. For this, add four sterile coverslips to four wells of 6 well plate using forceps.
Note: Copper is essential for tyrosinase activity in melanocytes and addition of copper to the fixed cells will allow visualizing tyrosinase activity in the secretory compartments such as endosomes and Golgi and enhance its activity in the melanosomes (Setty et al., 2008). - Coat the coverslips with a thin layer of Matrigel using 0.1% Matrigel matrix solution and dry the coverslips at room temperature for 15-30 min.
- Wash the coverslips thrice with 1x PBS (2-3 ml each, room temperature).
- Seed the mouse melanocytes approximately 0.5-0.8 x 106 in each well of 6 well plate or 60-70% confluence in any dish and incubate at 37 °C in 10% CO2 incubator.
- Fix the cells after 24 h with 4% formaldehyde in 1x PBS (room temperature) for 30 min.
- Wash the cells thrice with 1x PBS (room temperature).
- Incubate the cells with the following freshly prepared solutions at 37 °C for 2 h.
a.1x PBS and/or 0.1% D-DOPA in 1x PBS (negative control)
b.0.1% L-DOPA in 1x PBS
c.0.1% L-DOPA in 1x PBS with 20 μM copper sulphate (co-factor for tyrosinase) - Remove the solutions and repeat the incubation with the respective solutions.
Note: L-DOPA in PBS or water undergoes auto-oxidation and turns black after few hours. Moreover, this oxidation results in loss of active L-DOPA substrate in the solution and thus, repetition of this step with freshly made solutions will improve the activity of tyrosinase. - Wash the cells thrice with 1x PBS.
- Mount the coverslips with mounting medium on glass slides and image under the bright field microscope (Figure 1).
- Keep the bright field exposure time and settings identical for all the samples (Figure 1).
- Compare the bright field images of L-DOPA or L-DOPA+copper treated melanocytes with 1x PBS or D-DOPA. The images can be analyzed for differences in signal intensity and number of pigment granules, as well as their distribution within the cells.
Note: The increase in pigment granule number and intensity in melanocytes treated with L-DOPA or L-DOPA+copper (observe higher granule intensity compared to L-DOPA) is indicative to the presence of active tyrosinase in both intracellular vesicles and endocytic organelles including melanosomes (Setty et al., 2008).
Representative data
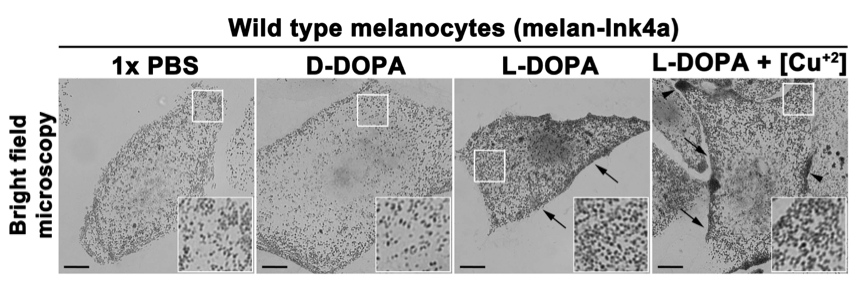
Figure 1. In vitro intracellular tyrosinase activity in wild type mouse melanocytes. Freshly fixed mouse melanocytes on glass coverslips were incubated with 1x PBS, D-DOPA (negative control) or L-DOPA with or without copper sulphate (20 μM) for 4 h. Images were captured at identical camera setting in a bright field mode on inverted fluorescence microscope. Arrows indicate the increased tyrosinase activity in cells treated with L-DOPA (with or without copper). Arrowheads point to the regions with enhanced tyrosinase activity in cells treated with L-DOPA+copper. Scale bars, 10 μm and insets, 2.5 times of the white boxed areas. Note that the melanin deposits are increased in cells treated with L-DOPA (with or without copper) compared to D-DOPA or 1x PBS incubated cells.
Notes
- Prepare D-DOPA and L-DOPA solutions always fresh, just before the incubation of cells.
- Use the freshly formaldehyde fixed melanocytes for the assay.
Recipes
- 1x PBS
137 mM NaCl
2.7 mM KCl
10 mM Na2HPO4
2 mM KH2PO4 (pH 7.4)
Sterilized using 0.22 μm filtration system and stored at room temperature (25 °C) - Acid wash and sterilization of coverslips
- Incubate the coverslips (approximately 100 in number) in 50 ml of HNO3 and HCl mixture (in 2:1 ratio) for 2 h at room temperature (25 °C).
- Wash the coverslips with deionized water for 3-4 times (50-100 ml each time) or until the pH of incubated water reached to 7.
- Wash the coverslips with 70% alcohol, dry in hot-air-oven (maintained at 50-60 °C) for overnight and sterilize by autoclaving in a glass beaker.
- Store the coverslips at room temperature in biosafety cabinet for repeated use.
- 0.1% Matrigel matrix solution
- Suspend 10 μl of Matrigel matrix in 10 ml of ice cold RPMI-1640 media (without fetal bovine serum) and keep the solution on ice during the experiment.
- Coat the coverslips by adding 1 ml of solution to each well at room temperature.
- After 1-2 min, collect the matrix solution and store at 4 °C for reuse.
- Dry the coverslips at room temperature for 15-30 min in a tissue culture cabinet with a closed lid.
- Growth media
RPMI-1640 media
10% fetal bovine serum
1% L-Glutamine
1% Penicillin-Streptomycin (antibiotic)
Sterilized using 0.22 μm filtration system and stored at 4 °C - 4% Formaldehyde solution
Dilute 1.081 ml of Formaldehyde solution with 1x PBS to 10 ml and prepare the solution always fresh - 0.1% DOPA solution
Dissolve 10 mg of D- or L- DOPA in 10 ml of sterile 1x PBS and prepare the solutions always fresh
Acknowledgments
This protocol was modified from previously published reports (Zhao et al., 1994; Boissy et al., 1998). We thank E. V. Sviderskaya and D. C. Bennett for generous gift of wild type mouse melanocytes. This work was supported by Wellcome Trust-DBT India Alliance Senior Fellowship 500122/Z/09/Z to S. R. G. S. and a UGC fellowship 2120930821/2009 to R. A. J.
References
- Jani, R. A., Purushothaman, L. K., Rani, S., Bergam, P. and Setty, S. R. (2015). STX13 regulates cargo delivery from recycling endosomes during melanosome biogenesis. J Cell Sci 128(17): 3263-3276.
- Setty, S. R., Tenza, D., Sviderskaya, E. V., Bennett, D. C., Raposo, G. and Marks, M. S. (2008). Cell-specific ATP7A transport sustains copper-dependent tyrosinase activity in melanosomes. Nature 454(7208): 1142-1146.
- Sviderskaya, E. V., Kallenberg, D. M. and Bennett, D. C. (2010). The wellcome trust functional genomics cell bank: Holdings. Pigment Cell Melanoma Res 23: 147-150.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Jani, R. A., Nag, S. and Setty, S. R. G. (2016). Visualization of Intracellular Tyrosinase Activity in vitro. Bio-protocol 6(8): e1794. DOI: 10.21769/BioProtoc.1794.
Category
Biochemistry > Protein > Activity
Cell Biology > Cell staining > Protein
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link












