- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Immunofluorescent Staining of Mouse Intestinal Stem Cells
Published: Vol 6, Iss 4, Feb 20, 2016 DOI: 10.21769/BioProtoc.1732 Views: 35001
Reviewed by: Xuecai GeAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Protocol for the Implantation of Scaffolds in a Humanized Mouse Cutaneous Excisional Wound Healing Model
Dina Gadalla [...] David G. Lott
Sep 20, 2024 1457 Views

Preparing Chamber Slides With Pressed Collagen for Live Imaging Monolayers of Primary Human Intestinal Stem Cells
Joseph Burclaff and Scott T. Magness
Nov 20, 2024 2030 Views

Dissection and Whole-Mount Immunofluorescent Staining of Mouse Hind Paw Muscles for Neuromuscular Junction Analysis
Rebecca L. Simkin [...] James N. Sleigh
May 20, 2025 3943 Views
Abstract
Immunofluorescent staining of organoids can be performed to visualize molecular markers of cell behavior. For example, cell proliferation marked by incorporation of nucleotide (EdU), or to observe markers of intestinal differentiation including paneth cells, goblet cells, or enterocytes (see Figure 1). In this protocol we detail a method to fix, permeabilize, stain and mount intestinal organoids for analysis by immunofluorescent confocal microscopy.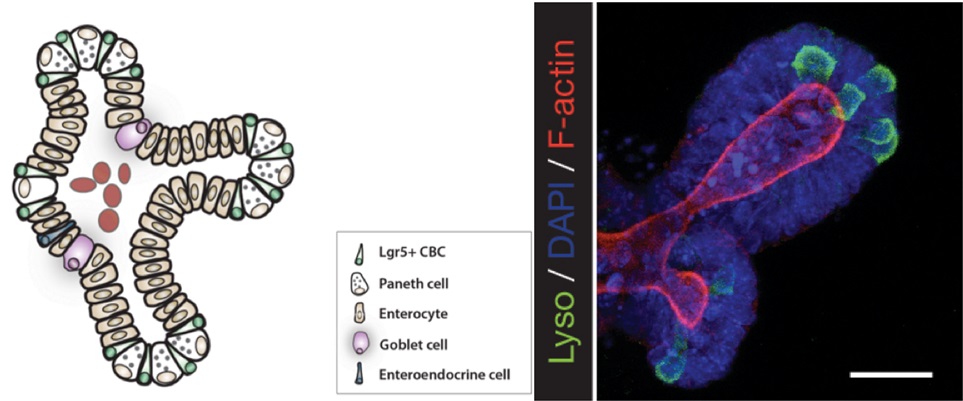
Figure 1. A schematic depicting a crypt-villus forming organoid, and visualization of Paneth cells by immunofluorescence staining. Left: Small intestinal organoids grow as crypt-villus structures that contain all of the multiple differentiated lineages of the intestine. Right: Immunofluorescent staining can be used to visualize individual cell types in the organoid. Here paneth cells are visualized by staining for lysozyme (“Lyso,” Green), which reveals Paneth cells located at crypt bases. F-Actin (Red) reveals crypt structure at the apical surface of the epithelium, and DAPI (Blue) reveals cell nuclei. Scale bar is 25 μm.
Materials and Reagents
- 8 well chamber slides (Thermo Fisher Scientific, Lab-TekTM, catalog number: 154532 )
- Cover Glass, Rectangular #1 (24 x 50 mm, 0.12-0.16 mm) (Corning, catalog number: 2975-245 )
- Primary antibodies
- Rabbit anti-KRT20 (1:200) (Cell Signaling Technology, catalog number: 13063 )
- Rabbit anti-Lysozyme (1:200) (Dako, catalog number: EC 3.2.1.17 )
- Rabbit anti-Muc2 (1:200)/ VHL Antibody (M-20) (Santa Cruz Biotechnology, catalog number: H-300, sc-1534 )
- Villin Antibody (C-19) (1:200) (Santa Cruz Biotechnology, catalog number: sc-7672 )
- Rabbit anti-KRT20 (1:200) (Cell Signaling Technology, catalog number: 13063 )
- Secondary antibodies:
- Goat Anti-rabbit 568 (1:500) (Thermo Fisher Scientific, Molecular Probes, catalog number: 11036 )
Note: Currently, it is “Thermo Fisher Scientific, NovexTM, catalog number: 11036”.
- Donkey Anti-goat 594 (1:500) (Thermo Fisher Scientific, Molecular Probes, catalog number: 11058 )
Note: Currently, it is “Thermo Fisher Scientific, NovexTM, catalog number: 11058”.
- Goat Anti-rat 488 (1:500) (Thermo Fisher Scientific, Molecular Probes, catalog number: a11006 )
Note: Currently, it is “Thermo Fisher Scientific, NovexTM, catalog number: a11006”.
- Goat Anti-rabbit 568 (1:500) (Thermo Fisher Scientific, Molecular Probes, catalog number: 11036 )
- Phosphate Buffered Saline (PBS) (Thermo Fisher Scientific, InvitrogenTM, catalog number: 10010023 )
Note: Currently, it is “Thermo Fisher Scientific, GibcoTM, catalog number: 10010023”.
- Paraformaldehyde (PFA) 16% Solution, EM Grade (Electron Microscopy Sciences, catalog number: 15710-S )
- Freshly prepared 4% PFA in 1x PME Buffer
- ProLong Gold Antifade Mountant (Thermo Fisher Scientific, Molecular Probes, catalog number: P10144 )
Note: Currently, it is “Thermo Fisher Scientific, ProLong®, catalog number: P10144”.
- Clear Nail Polish (available at local drug store)
- Optional
- Click-iT EdU Alexa Fluor 647 for Cell Proliferation (Thermo Fisher Scientific, Invitrogen, catalog number: C10340 )
Note: Currently, it is “Thermo Fisher Scientific, Molecular ProbesTM, catalog number: C10340”.
- Alexa Fluor 647 Phalloidin (Thermo Fisher Scientific, Molecular ProbeTM, catalog number: A22287 )
- BCIP/NBT Substrate Kit (Vector Laboratories, catalog number: SK-5400 )
- 1 μg/ml DAPI for nucleic acid staining (Sigma-Aldrich, catalog number: D9542 )
Note: Materials and equipment to grow organoids prior to fixation (see Isolation, culture, and maintenance of mouse intestinal stem cells)
- Click-iT EdU Alexa Fluor 647 for Cell Proliferation (Thermo Fisher Scientific, Invitrogen, catalog number: C10340 )
- Tris(hydroxymethyl)aminomethane (Sigma-Aldrich, catalog number: 252859 )
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S9888 )
- PIPES (Sigma-Aldrich, catalog number: P6757 )
- Magnesium chloride (MgCl2) (Sigma-Aldrich, catalog number: M8266 )
- Ethylenediaminetetraacetic acid (EDTA) (Sigma-Aldrich, catalog number: E9884 )
- TritonTM X-100 (Sigma-Aldrich, catalog number: X100 )
- TWEEN® 20 (Sigma-Aldrich, catalog number: P2287 )
- Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A2058 )
- Tris buffered saline (TBS) (see Recipes)
- 10x PME buffer (see Recipes)
- IF buffer (see Recipes)
- Blocking solution (see Recipes)
- Permeabilization solution (see Recipes)
Equipment
- Benchtop Multi-Purpose Rotator (Thermo Fisher Scientific, model: 2309 )
- Leica Inverted Confocal SP8 equipped with a White Light Laser, a Leica HyD Detector and the Leica Application Suite software
Software
- Leica Application Suite
Procedure
- Plate 40 μl of the matrigel containing organoids into 1 well each of an 8-well chamber-slide. After letting harden in the incubator for 10-15 min, add 400 μl of media to the well and change at least every two days. If analyzing proliferation by EdU incorporation, add 10 μM EdU to the wells 6 h prior to fixation.
- When ready for fixation, remove the media and add 300 μl freshly prepared 4% PFA in PME buffer (room temperature) for 20 min. For this step, and throughout the entire protocol, to change the solution in the well, carefully place a pipette tip into the side of the well and draw up the solution. Then carefully add the new solution down the side of the well. This is performed the same way when changing media (O’Rourke et al., 2016).
- Remove the fixative, wash once with IF buffer. Add 300 μl of Permeabilization Solution to the well to permeabilize for 20 min.
- Remove the solution, wash once with IF Buffer and then add Blocking Solution for 30 min at room temperature.
- Alternative step: To visualize enterocytes by alkaline phosphatase staining, wash the fixed cells twice with TBS and then incubate with 300 μl of the BCIP/NBT Substrate Kit Solution for 15 min in the dark (follow directions that accompany the kit to make the BCIP/NBT solution). The chambers are then washed twice with TBS and then imaged using bright field microscopy. An example is shown in Figure 2.
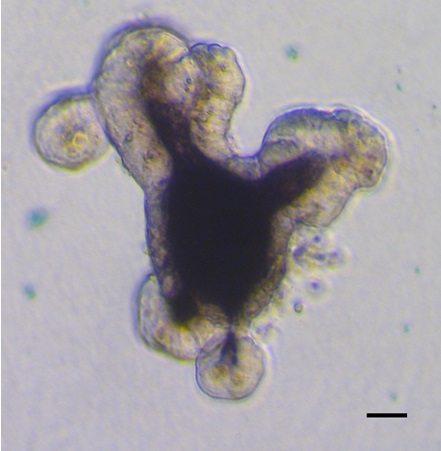
Figure 2. A brightfield image of a small intestinal organoid showing strong alkaline phosphatase activity (indicating the presence of differentiated enterocytes). Scale bar is 50 μm.
- Alternative step: Prior to primary antibody incubation, the samples can be processed for EdU visualization via Click-It chemistry using the directions provided with the kit. At this point follow the Invitrogen Protocol step 4.3 and finish with 4.7. Then, if required, incubate your sample in 150 μl of primary antibody as in step 7. For an example of an EdU and Keratin 20 stained small intestinal organoid, see Figure 3. Be sure to protect the sample from light from this step forward.
- Prepare the primary antibody in blocking solution and add 150 μl to each well. See Notes on Materials for antibodies we have validated. Let the chamber-slide sit overnight in a large, humidified chamber at 4 °C. Do not leave the chamber-slide lid on the chamber-slide as it can cause liquid to be pulled out of the chamber.
- After overnight incubation, wash each well 3 times for 5 min each with 300 μl IF buffer. Place the chamber slide on a benchtop orbital shaker set to 60 RPM during each wash.
- Prepare secondary antibody in Blocking Solution. For a list of secondary antibodies we have validated, see Note 4. Add 150 μl of the solution to the well and let sit at room temperature for 1 h, being sure to protect from light. Optionally, a phalloidin-conjugated fluorophore can be added to the secondary solution to visualize F-actin polymers.
- Optional step: To visualize DNA/nuclei, prepare a solution of DAPI (1 μg/ml) in IF buffer and add 300 μl to each well for 5 min. Then proceed with washing (step 11).
- After secondary incubation, wash each well 3 times with 300 μl IF buffer as in step 8.
- Remove the IF buffer and detach the chambers using the removal kit that accompanies the chamber slides.
- Using a p200 pipette tip with the tip cut off (see Note 6), add a small amount of ProLong Gold antifade mounting medium over each specimen (~20-30 μl each).
- Place a cover slip over the specimen, avoiding bubbles. Use clear nail polish to seal the sides of the cover glass to the slide. Let harden overnight at room temperature in the dark. After imaging, the slide can be stored at 4 °C for at least 6 months with minimal loss of fluorescence.
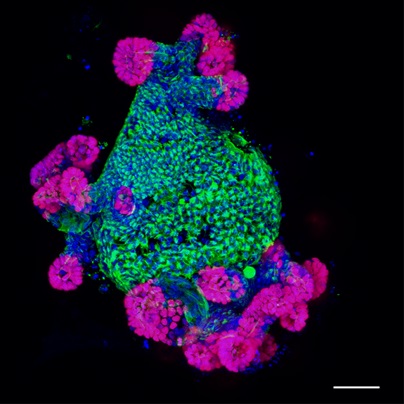
Figure 3. Immunofluorescent image of a small intestinal organoid showing markers of proliferation (EdU, Red), differentiation (Krt20, Green) and nuclei (blue). Scale bar is 50 μm.
Notes
- During washes you will need to pipette liquid in and out of the chamber wells while carefully avoiding the organoids. A p1000 pipette tip with a tapered end can be helpful for this.
- We have had success with the Click-iT Edu Alexa Fluor 647 kit from Invitrogen, but note this kit is available with a wide range of fluorophores, which can be used to prevent overlap with any fluorescent protein that may be native to your cells. Note that this kit will also destroy F-actin, which prevents visualization of the actin cytoskeleton by Phalloidin conjugated fluorophores, as well as native GFP.
- We have validated the following primary antibodies for use in this assay: Rabbit anti-KRT20 (1:200), rabbit anti-Lysozyme (1:200), rabbit anti-Muc2 (1:200) and goat anti-Villin (1:200).
- We have validated the following secondary antibodies for use in this assay: Anti-rabbit 568 (1:500), anti-goat 594 (1:500) and anti-rat 488 (1:500).
- After the fixation and permeabilization steps, most of the Matrigel will have degraded, leaving the organoids to rest on the bottom of the well. This is true for small and large intestinal organoids grown for 4-6 days in culture prior to fixation, perhaps due to the production of intestinal proteases. We have noticed that shorter culture times, or different organoid cell types, can prevent this.
- When using the p200 to pipette the mounting medium over the cells, it is helpful to first use scissors to cut the tip at the tapered end to make it larger in diameter, which will avoid bubbles when pipetting the solution.
- We have had the best results imaging these samples using a confocal microscope. Specifically, we use a Leica Inverted Confocal SP8 equipped with a White Light Laser, a Leica HyD Detector and the Leica Application Suite software. This setup allows the excitation laser to be set to any wavelength between 470 and 670 nm, in addition to a 405 laser for DAPI excitation and the standard argon laser lines of 458, 476, 488, 496 and 514 nm. This means one can optimally excite and cleanly separate any fluorophores. The SP8 is also equipped with gating technology, which significantly eliminates autofluorescence.
- We have used the HC PL APO 20x .7NA IMM and the HCX PL APO 40x 1.1NA objectives for imaging these samples.
- For an example of an immunofluorescent image obtained using this microscope setup (see Figure 3).
Recipes
- Tris buffered saline (TBS)
25 mM Tris
0.15M NaCl
Final pH of solution should be 7.2 to 7.5
- 10x PME buffer
500 mM PIPES
25 mM MgCl2
50 mM EDTA
- IF buffer
PBS containing:
- 0.2% Triton X-10
- 0.05% Tween
- 0.2% Triton X-10
- Blocking solution
IF Buffer containing:
1% bovine serum albumin (BSA)
- Permeabilization solution
PBS containing
0.5% Triton X-100
Acknowledgments
We thank members of the Lowe Lab, as well as the Molecular Cytology Core Facility at Memorial Sloan Kettering Cancer Center, for helpful input. This work was supported by a program project grant from the NIH/ NCI (CA-013106). L. E. D. was supported by a National Health and Medical Research Council (NHMRC) Overseas Biomedical Fellowship and a K22 Career Development Award from the NCI/NIH (CA-181280-01). K. P. O. was supported by a Medical Scientist Training Program grant from the National Institute of General Medical Sciences of the NIH under award number T32GM07739 to the Weill Cornell/Rockefeller/Sloan-Kettering Tri-Institutional MD-PhD Program. S. W. L. is the Geoffrey Beene Chair of Cancer Biology and an investigator of the Howard Hughes Medical Institute.
References
- Dow, L. E., O'Rourke, K. P., Simon, J., Tschaharganeh, D. F., van Es, J. H., Clevers, H. and Lowe, S. W. (2015). Apc restoration promotes cellular differentiation and reestablishes crypt homeostasis in colorectal cancer. Cell 161(7): 1539-1552.
- O’Rourke, K. P., Ackerman, S., Dow, L. E. and Lowe, S. W. (2016). Isolation, culture, and maintenance of mouse intestinal stem cells. Bio-protocol 6(4): e1733.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
O’Rourke, K. P., Dow, L. E. and Lowe, S. W. (2016). Immunofluorescent Staining of Mouse Intestinal Stem Cells. Bio-protocol 6(4): e1732. DOI: 10.21769/BioProtoc.1732.
Category
Stem Cell > Adult stem cell > Intestinal stem cell
Cell Biology > Tissue analysis > Tissue staining
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link