- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Mouse Oocyte Isolation, Cultivation and RNA Microinjection
Published: Vol 6, Iss 3, Feb 5, 2016 DOI: 10.21769/BioProtoc.1729 Views: 17803
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Identification and Sorting of Adipose Inflammatory and Metabolically Activated Macrophages in Diet-Induced Obesity
Dan Wu [...] Weidong Wang
Oct 20, 2025 2243 Views

Selective Enrichment and Identification of Cerebrospinal Fluid-Contacting Neurons In Vitro via PKD2L1 Promoter-Driven Lentiviral System
Wei Tan [...] Qing Li
Nov 20, 2025 1343 Views

Revisiting Primary Microglia Isolation Protocol: An Improved Method for Microglia Extraction
Jianwei Li [...] Guohui Lu
Dec 5, 2025 1475 Views
Abstract
Mammalian oocyte is a highly specialized cell, characterized by synthesis and storage of maternal proteins and RNAs that contributes to the meiotic cell cycle and early embryo development. The fully grown oocyte is transcriptionally quiescent and utilizes only transcripts synthesized and stored during the growing phase. Mouse oocytes are often used as a mammalian model for the study of molecular biology of the cell or biomedical research. Microinjection technique is a useful tool to deliver RNA coding for fluorescently tagged proteins to determine their subcellular localization or function, delivering biosensors for the study of various metabolic pathways or downregulation of specific targets by RNAi or oligo morpholinos to study gene function. Here, we describe a protocol for isolation, cultivation and microinjection of oocytes that might contribute to research or educational purposes.
Keywords: OocyteMaterials and Reagents
- Petri dishes (90 mm) (GAMA GROUP, catalog number: 400974 )
- Needles Omnifix F Duo (B. Braun Melsungen AG, catalog number: 9161465V )
- Petri dishes 3 ml, 8.8 cm2 (Thermo Fisher Scientific, catalog number: 153066 )
- Holding micropipette (Microtech IVF, catalog number: 001-120-30 )
- Borosilicate Thin Wall with Filament, 1.0 mm OD 0.78 mm ID, 150 mml (Harvard Apparatus, catalog number: 300039 )
- Microloader TM, tip for filling Femtotips and other glass microcapillaries, Sterile, 0.5-20 µl, 100 mm (Eppendorf, catalog number: 5242956003 )
- Cultivation medium M16 (Merck Millipore Corporation, catalog number: MR016D )
- 4 well cell culture plate (SPLLIFESCIENCES, catalog number: 30004 )
- µ-Slide 4 Well Glass Bottom (Ibidi, catalog number: 80427 )
- Nunc Lab-Tek II Chamber Slide System (Thermo Fisher Scientific, catalog number: 154534 )
- Capillaries for oocyte manipulation with tip 100 µm in diameter
- mMESSAGE mMACHINE Kit (Life Technologies, catalog number: AM1344 )
Note: Currently, it is “Thermo Fisher Scientific, Ambion™, catalog number: AM1344”. - Stimulated mouse (Mus musculus, CD1) at least 6 weeks old; stimulation via pregnant mare serum gonadotropin (PMSG)-Folligon (MSD Animal Health) and human chorionic gonadotropin (hCG) (Sigma-Aldrich)
- 3-isobutyl-1-methylxanthine (IBMX) (Sigma-Aldrich, catalog number: 28822584 )
- Poly(A) Tailing Kit (Life Technologies, catalog number: AM1350 )
Note: Currently, it is “Thermo Fisher Scientific, Ambion™, catalog number: AM1350”. - RNeasy Mini Kit (QIAGEN, catalog number: 74104 )
- Mineral oil (Sigma-Aldrich, catalog number: M8410 )
- RNase-free water (Life Technologies, Ambion®, catalog number: AM9932 )
Note: Currently, it is “Thermo Fisher Scientific, Ambion™, catalog number: AM9932”. - Dyes for monitoring fluid injection into oocyte,
- NaCl
- KCl
- CaCl2.2H2O
- KH2PO4
- MgSO4.7H2O
- Glucose
- 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)
- Polyvinyl alcohol (PVA)
- Destilated water
- Bovine serum albumin (BSA)
- Transfer medium (see Recipes)
Equipment
- Incubator Hera Cell 150 (Heraeus Holding)
- Stereo microscope Stemi 2000 (ZEISS)
- NanoDrop ND-1000 (Thermo Fisher Scientific)
- Centrifuge 5418 (Eppendorf)
- Pulling capillaries (Sutter Instrument Company, model: P-97 )
- Microforge for bending capillaries (NARISHIGE Group, model: MF-79 )
- Inverted microscope (OLYMPUS, model: CKX41 and Leica, model: DMI 6000B )
- Channel Pressure Injector (MicroData Instrument, model: PM2000B 4 )
- Joystick MIS-5000 Series Microinjection Manipulation Systems (Burleigh)
- Pressurized nitrogen gas or FemtoJet (Eppendorf)
- Confocal microscope (Leica, model: SP5 )
- EMBL stage incubator
- Water corrected objectives HCX PL APO 20x/0.7 IMM CORR λBL and HCX PL APO 40/1.1
Software
- Image J (http://rsbweb.nih.gov/ij)
Procedure
- Preparation of RNA/morpholinos
- Perform the in vitro RNA transcription of sequence of your interest (your RNA, or morpholino oligo for blocking selected specific RNA targets) using mMESSAGE mMACHINE Kit and add poly(A) tail with Poly(A) Tailing Kit.
- Purify the RNA sample by RNeasy Mini Kit.
- Measure RNA concentration by NanoDrop.
- Store the RNA in -80 °C before usage.
- Prepare working solution with a 20-50 ng/µl concentration of RNA or 1-10 µM concentration of morpholino. Dilute in RNase free water. Important: Centrifuge for 5 min/17,000 x g and use supernatant only to get rid of particles which then might seal the tip of micropipette.
- Keep working solution on ice.
- Perform the in vitro RNA transcription of sequence of your interest (your RNA, or morpholino oligo for blocking selected specific RNA targets) using mMESSAGE mMACHINE Kit and add poly(A) tail with Poly(A) Tailing Kit.
- Micropipette preparation
- Set parameters on micropipette puller: heat 300, pull 80, velocity 70, time 150.
- Fix the capillary into the groove, pull firmly the holder, close the cover and press PULL. Thus you get the capillary with thin end suitable for microinjection (see Video 1).
- Then fix the capillary into the manipulator MF-79. Magnify and focus the end of the capillary and close it to the hot fiber which bends the capillary into the proper angle, approximately 30 degrees (see Video 1).
- Fill the prepared injection capillary with working solution (approx 1.5 µl) using microloader tip.Video 1. Micropipette preparation
- Set parameters on micropipette puller: heat 300, pull 80, velocity 70, time 150.
- Oocyte isolation and cultivation
- Obtain mouse ovaries from laboratory mice (Mus musculus, CD1) at least 6 weeks old (from 6 to 10 weeks old), which were stimulated by Folligon (PMSG; 5 IU per one mouse) 46 h before collection to get oocytes in the stage of germinal vesicle (GV stage). For zygotes, administer hCG (5 IU per one mouse) 48 h after PMSG and mate stimulated females with males. Isolate zygotes 17 h after mating.
- Euthanize the mice by cervical dislocation. Cervical dislocation is the recommended method of mice euthanasia due to its speed and reliability (of course after the acquisition of proper technique). Moreover any chemicals that could potentially affect the experiment are not added.
- Remove the ovaries and clean them from the fat tissue.
- Isolate oocytes immediately after collection ovaries using two needles: disrupt the follicles in transfer medium (TM) supplemented with IBMX (100 µM) (see Video 2).
- Transfer isolated oocytes into the cultivation dish with M16 medium with IBMX (100 µM) and incubate at 37.5 °C, 5% CO2. The dishes with medium have to be equilibrated before for at least 2 h in 37.5 °C, 5% CO2.
- After 15 min in the incubator, denude the oocytes by gentle pipetting (suction and discharge several times) the medium in the well. The pipetting will mechanically remove cumulus cells from the oocyte. Then let the oocytes in the incubator for other 15 min.
- Then select the best oocytes visually (rounded, nucleolus in the middle, fully grown oocytes ~ 70 µm in diameter; Figure 1). Video 2. Oocyte isolation
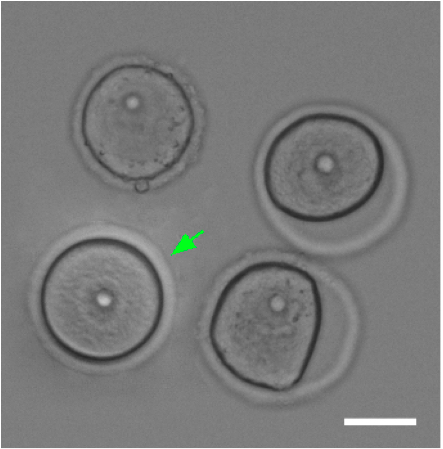
Figure 1. Comparison of good and bad oocytes. Green arrow marks a good oocyte. Scale bar 40 µm
- Obtain mouse ovaries from laboratory mice (Mus musculus, CD1) at least 6 weeks old (from 6 to 10 weeks old), which were stimulated by Folligon (PMSG; 5 IU per one mouse) 46 h before collection to get oocytes in the stage of germinal vesicle (GV stage). For zygotes, administer hCG (5 IU per one mouse) 48 h after PMSG and mate stimulated females with males. Isolate zygotes 17 h after mating.
- Microinjection
- Turn on the microscope, microinjector, joystick and the gas (pressure up to 500 kPa). Set the injection pressure: FILL 0.1 psi, INJ 10.1 psi, BAL 2.9 psi, HOLD 14.5 psi.
- Put the holding capillary and the loaded injection capillary to the relevant holders and close them slowly to the petri dish with a drop of TM + IBMX and the oocytes.
- Then immediately inject: Hold the oocyte using the holding capillary (holding capillary creates a negative pressure by which the oocyte is held) and inject ~5 pl of your working solution from the injection capillary into oocyte, not close to the nucleus (Figure 2).
- Transfer oocytes with constructs to incubator in M16 + IBMX.
- To verify the success of the microinjection use fluorescent microscopy.
- See also Video 3.
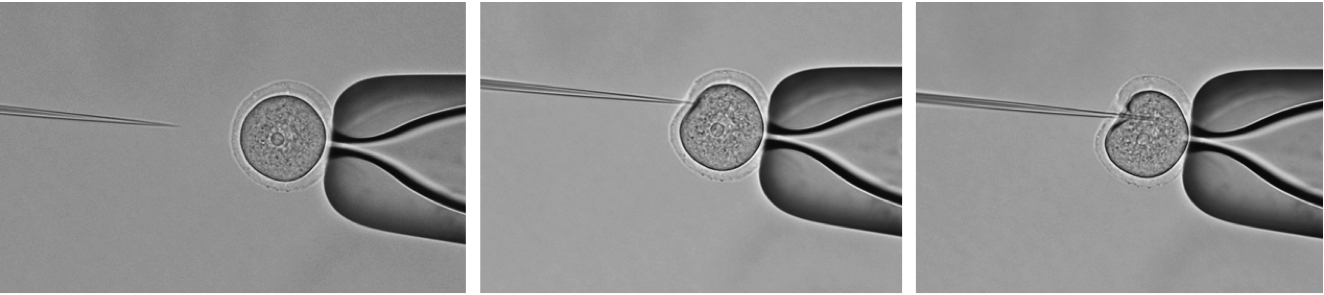
Figure 2. Microinjection. Inject working solution not close to the nucleus.Video 3. Microinjection
- Turn on the microscope, microinjector, joystick and the gas (pressure up to 500 kPa). Set the injection pressure: FILL 0.1 psi, INJ 10.1 psi, BAL 2.9 psi, HOLD 14.5 psi.
- Live-cell imaging
- 1-2 h after microinjection wash the oocytes from IBMX in TM and then in M16.
- Transfer the oocytes into 2 µl of M16 covered with oil in Lab-tek Chamber Slide System. The dish with medium has to be equilibrated in 37.5 °C, 5% CO2. Raised coverslip is not suitable. We also use dishes from Ibidi (No. 1.5 H), similar to Labtek. Or, for cell culture (not for oocytes) you can choose for example a dish from µ-Slide I Luer Family.
- Place your sample in the confocal microscope equipped with EMBL stage incubator (37.5 °C, 5% CO2). Be sure that the incubator is closed.
- Turn on the appropriate lasers and set the software for video capturing (image each 5-15 min). We usually let the mouse oocytes cultivate overnight, but cultivations of embryos over a week long were published, depending on the chemistry of respective dyes, bleaching etc.
- Assembly the movie using Image J (http://rsbweb.nih.gov/ij).
- See also Figure 3 and Video 4 showing visualization of meiotic maturation of mouse oocyte using H2B-GFP mRNA.
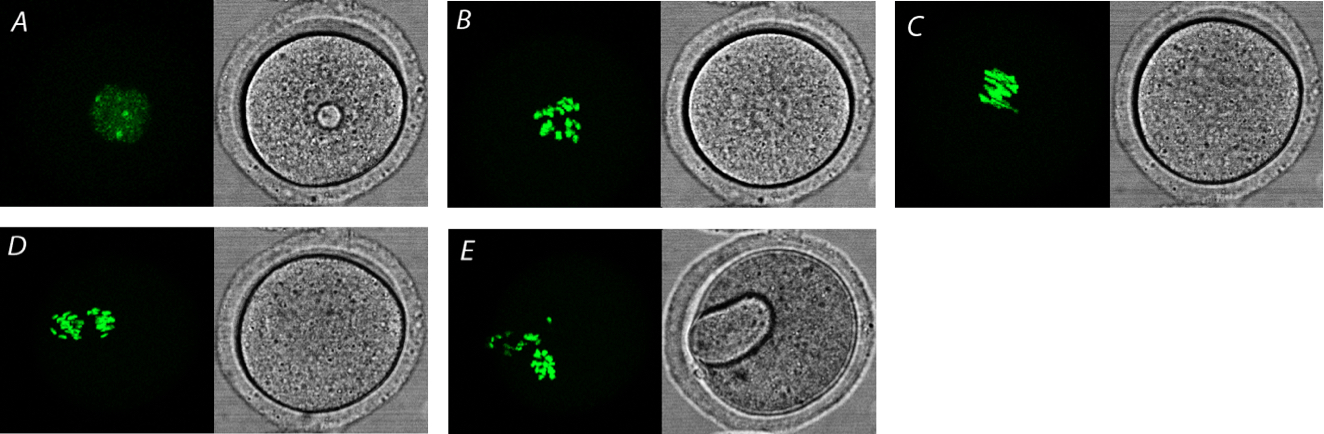
Figure 3. Visualization of meiosis in mouse oocyte. Visualization using microinjected H2B-GFP (green). A. Fully grown oocyte in GV stage. B. Oocyte after nuclear envelope breakdown (NEBD) and typical chromosomal spread. C. Oocyte in metaphase I (MI) stage. D. Oocyte in anaphase I. E. Oocyte after first meiotic division with extruded first polar body.Video 4. Mouse oocytes during meiotic maturation with microinjected H2B-GFP. Control oocytes are not microinjected (yellow arrows), H2B-GFP is shown in green. Growing oocyte (blue arrow) has no meiotic competence and remains in GV stage.
- 1-2 h after microinjection wash the oocytes from IBMX in TM and then in M16.
Recipes
- Transfer medium
NaCl 12.8 g
KCl 0.8 g
CaCl2.2H2O 0.6 g
KH2PO4 0.14 g
MgSO4.7H2O 0.194 g
Glucose 4 g
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) 4 g
Polyvinyl alcohol (PVA) 2 g
Distilled water 2 L
Bovine serum albumin (BSA) 5 g
Acknowledgments
This work was supported by research grant GACR 13-12291S to Andrej Susor. The original work was published in Karabinova et al., (2011) and Susor et al., (2015). Thanks to colleagues from Laboratory of Biochemistry and Molecular Biology of Germ Cells for support.
References
- Gagnon, J. A. and Mowry, K. L. (2010). Visualizing RNA localization in Xenopus oocytes. J Vis Exp (35): e1704.
- Karabinova, P., Kubelka, M. and Susor, A. (2011). Proteasomal degradation of ubiquitinated proteins in oocyte meiosis and fertilization in mammals. Cell Tissue Res 346(1): 1-9.
- Layden, M. J., Rottinger, E., Wolenski, F. S., Gilmore, T. D. and Martindale, M. Q. (2013). Microinjection of mRNA or morpholinos for reverse genetic analysis in the starlet sea anemone, Nematostella vectensis. Nat Protoc 8(5): 924-934.
- Susor, A., Jansova, D., Cerna, R., Danylevska, A., Anger, M., Toralova, T., Malik, R., Supolikova, J., Cook, M. S., Oh, J. S. and Kubelka, M. (2015). Temporal and spatial regulation of translation in the mammalian oocyte via the mTOR-eIF4F pathway. Nat Commun 6: 6078.
- Yuan, S. and Sun, Z. (2009). Microinjection of mRNA and morpholino antisense oligonucleotides in zebrafish embryos. J Vis Exp (27): e1113.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Tetkova, A. and Hancova, M. (2016). Mouse Oocyte Isolation, Cultivation and RNA Microinjection. Bio-protocol 6(3): e1729. DOI: 10.21769/BioProtoc.1729.
Category
Cell Biology > Cell isolation and culture > Cell isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link












