- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Infection of Human Hepatocyte-chimeric Mice with HBV and in vivo Treatment with εRNA
Published: Vol 6, Iss 2, Jan 20, 2016 DOI: 10.21769/BioProtoc.1718 Views: 7898
Reviewed by: Jia LiRamalingam BethunaickanAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Visualizing Hypoxia in a Murine Model of Candida albicans Infection Using in vivo Biofluorencence
José Pedro Lopes and Constantin F. Urban
Aug 5, 2019 5112 Views

RNA Extraction from Ears and Draining Lymph Nodes of Mice Infected with Leishmania amazonensis
Emilie Giraud and Evie Melanitou
Jun 5, 2020 5515 Views

TetR Regulated in vivo Repression Technology to Identify Conditional Gene Silencing in Genetically Engineerable Bacteria Using Vibrio cholerae Murine Infections as Model System
Franz G. Zingl [...] Stefan Schild
Oct 5, 2020 3749 Views
Abstract
Hepatitis B virus (HBV) can cause both acute and chronic disease in human liver with potentially high risk of cirrhosis and liver cancer. The host range of non-human primates susceptible to this virus is limited. Therefore, experimental studies with human hepatocyte-chimeric mice provide an invaluable source of information regarding the biology and pathogenesis of HBV. This section describes the protocol for infection of the human hepatocyte-chimeric mice with HBV. In addition, it has recently been shown that HBV replication can be suppressed by exogenous expression of viral epsilon RNA (εRNA; Sato et al., 2015), which serves as an encapsidation signal (Bartenschlager et al., 1992). Based upon this finding, we also describe the protocol for the liposome-mediated delivery of a plasmid encoding εRNA to liver in these chimeric mice.
Keywords: Hepatitis B virusMaterials and Reagents
- 0.1-10 μl pipet tips (Thermo Fisher Scientific, catalog number: QSP# TF104 )
- 1-200 μl and 100-1,000 μl pipet tips (Corning, catalog number: 4845 and 4846 , respectively)
- 0.2 ml 8 strips PCR tubes and caps (NIPPON Genetics, catalog number: FG-028DC )
- 1.5 ml and 2.0 ml microcentrifuge tubes (Corning, catalog number: MCT-150-A and MCT-200-C , respectively)
- 15 ml and 50 ml centrifuge tubes (Corning, catalog number: 352096 and 352070 , respectively)
- 96-well fast plate (NIPPON Genetics, catalog number: 38801 )
- 1 ml syringe (MonotaRO Co., NIPRO Genetics, catalog number: 08-010 )
- Human hepatocyte-chimeric mice (PhoenixBio Co.)
Note: Chimeric mice are intravenously infected with 100 μl of HBV-C in saline solution (106 copies per mouse) derived originally from patient with chronic hepatitis (Sugiyama et al., 2006). - HBV (genotype C; HBV-C) (Dr. Yasuhito Tanaka, Department of Virology and Liver Unit, Nagoya City University Graduate School of Medical Sciences, Nagoya, Japan; Sugiyama et al., 2006)
- Sodium chloride (Nacalai tesque, catalog number: 31320-05 )
- YSK lipid (a pH-sensitive cationic lipid) (Faculty of Pharmaceutical Sciences, Hokkaido University, Sapporo, Hokkaido, Japan) (Sato et al., 2012)
- Cholesterol (Avanti Polar Lipid, catalog number: 57-88-5 )
- 1, 2-dimyristoyl-sn-glycerol methoxypolyethyleneglycol (PEG-DMG) (NOF Corporation, catalog number: GM-020 )
- Nuclease free-H2O
- Citrate buffer (12.5 mM citrate, 500 mM NaCl)
- pCpGfree-mcs vector (Invivogen)
- Primers for vector construction
pLKO.1 Fw SpeI: CCCACTAGTTTTCCCATGATTCCTTCATATTT
pLKO.1 Rv BglII: CCCAGATCTAAAATTGTGGATGAATACTGCC - TaqMan Universal PCR Master Mix (Life Technologies, catalog number: 4304437 )
Note: Currently, it is “Thermo Fisher Scientific, Applied Biosystems™, catalog number: 4304437”.
- Primers and probe for quantification of HBV DNA from HBV-infected chimeric mice sera:
Forward Primer: SF2: 5’-CTTCATCCTGCTGCTATGCCT-3’
Reverse Primer: SR2: 5’-AAAGCCCAGGATGATGGGAT-3’
Probe: SP2: FAM-ATGTTGCCCGTTTGTCCTCTAATTCCAG-TAMRA - MISSION® pLKO.1-puro Empty Vector Control Plasmid DNA (Sigma-Aldrich, catalog number: SHC001 )
- DNA oligo nucleotides (5’-3’)
Sense: CCGGTGTACATGTCCCACTGTTCAAGCCTCCAAGCTGTGCCTTGGGTGGCTTTGGGGCATGGACATTTTTG
Antisense:
AATTCAAAAATGTCCATGCCCCAAAGCCACCCAAGGCACAGCTTGGAGGCTTGAACAGTGGGACATGTACA - Oligo nucleotides (see Recipes)
Equipment
- Biosafety hood in a biosafety level 3 (BSL3) facility (HITACHI, catalog number: SCV-1303 ECIIB )
- Pipettes (PIPETMAN P2, P20 and P1000) (Gilson Scientific, catalog number: F144801 , F123600 and F123602 , respectively)
- qPCR adhesive seal (NIPPON Genetics, catalog number: 4Ti-0560 )
- Applied Biosystems Veriti Thermal Cycler (Life Technologies, catalog number: 4375786 )
Note: Currently, it is “Thermo Fisher Scientific, Applied Biosystems™, catalog number: 4375786”. - ABI StepOnePlusTM Real-Time PCR Systems (Life Technologies, catalog number: 4379216 )
Note: Currently, it is “Thermo Fisher Scientific, Applied Biosystems™, catalog number: 4379216”.
Procedure
- εRNA-MEND preparation
- The oligo nucleotides are annealed and inserted into an AgeI-EcoRI doubly digested pLKO.1-puro vector. With the ligated vector, the U6 promoter and εRNA-coding fragment are amplified through PCR by using a pair of primers (pLKO.1 Fw SpeI: CCCACTAGTTTTCCCATGATTCCTTCATATTT; pLKO.1 Rv BglII: CCCAGATCTAAAATTGTGGATGAATACTGCC), the PCR product is digested with SpeI and BglII and inserted into pCpGfree-mcs vector. This final construct is hereinafter called p-εRNA.
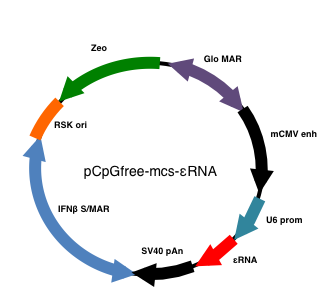
Figure 1. A plasmid map of U6 promoter-driven εRNA expression vector (p-εRNA) - The preparation of the p-εRNA loaded in a liposome carrier is based on the procedure that was previously described (Sato et al., 2012): p-εRNA or empty pCpGfree-mcs vector is formulated into lipid nanoparticles (MEND). 80 μg of p-εRNA or empty vector (in H2O) is dissolved in 120 μl of citrate buffer (12.5 mM citrate, 500 mM NaCl). Addition of this solution to 480 μl of the tertiary butanol containing YSK lipid (2,100 nmol), cholesterol (900 nmol), and 1, 2-dimyristoyl-sn-glycerol, methoxypolyethyleneglycol (150 nmol) leads to spontaneous formulation of liposomal particles (εRNA-MEND or control-MEND). The prepared εRNA-MEND is stocked at 4 °C until use.
- The oligo nucleotides are annealed and inserted into an AgeI-EcoRI doubly digested pLKO.1-puro vector. With the ligated vector, the U6 promoter and εRNA-coding fragment are amplified through PCR by using a pair of primers (pLKO.1 Fw SpeI: CCCACTAGTTTTCCCATGATTCCTTCATATTT; pLKO.1 Rv BglII: CCCAGATCTAAAATTGTGGATGAATACTGCC), the PCR product is digested with SpeI and BglII and inserted into pCpGfree-mcs vector. This final construct is hereinafter called p-εRNA.
- Infection of human hepatocyte-chimeric mice with HBV
Note: All procedures involving the manipulation of HBV infectious materials should be conducted within biological safety cabinets (BSL3).
Chimeric mice are intravenously infected with 100 μl of HBV-C in saline solution (106 copies per mouse) derived originally from patient with chronic hepatitis (Sugiyama et al., 2006). Three weeks after HBV infection, the sera are prepared by collecting blood samples from the tail vein and the efficiency of infection is confirmed by measuring the number of viral genome copies in the sera of HBV-infected chimeric mice by qPCR analysis as below:- Prepare HBV DNA standard sample:
The HBV plasmid (pUC19-HBV, genotype A) is subjected to a 10-fold serial dilutions in Nuclease free-H2O ranging from 1 x 103 to 1 x 109 copies/ml, and use 10 μl of this diluted sample (ranging from 1 x 10 to 1 x 107 copies/assay) as standard to quantification of HBV DNA. - Set up qPCR reaction mixtures as follows (for one sample):
DNA samples from sera of HBV-infected chimeric mice:Nuclease free-H2O 6 μl TaqMan Universal PCR Master Mix 12.5 μl Probe SP2 (10 μM) 0.5 μl Forward primer SF2 (10 μM) 0.5 μl Reverse primer SR2 (10 μM) 0.5 μl HBV DNA sample (from sera) 5 μl 25 μl
Standard DNA samples:Nuclease free-H2O 1 μl TaqMan Universal PCR Master Mix 12.5 μl Probe SP2 (10 μM) 0.5 μl Forward primer SF2 (10 μM) 0.5 μl Reverse primer SR2 (10 μM) 0.5 μl Standard DNA: 10 μl Total 25 μl
Note: Use 10 μl of nuclease free-H2O instead of standard DNA as negative control. Each sample is prepared in triplicate. - Start the real-time PCR following the program as below:
Holding stage 95 °C 10 min 1 cycle Cycling stage 95 °C 15 sec 45 cycles 60 °C 1 min Data collection Final holding stage 4 °C ∞
- Prepare HBV DNA standard sample:
- In vivo treatment with εRNA
At 4-week postinfection, εRNA-MEND or control-MEND, is administered intravenously at a dose of 0.5 mg/kg of body weight (n=3 per group) every two days for 14 days. Serum and liver samples can be subjected to qPCR for the quantification of DNA copy numbers of HBV or other analyses.
Recipes
- DNA oligonucleotides used for the p-εRNA plasmid construction
Sequence (5’→3’) Sense CCGGTGTACATGTCCCACTGTTCAAGCCTCCAAGCTGTGCCTTGGGTGGCTTTGGGGCATGGACATTTTTG Antisense AATTCAAAAATGTCCATGCCCCAAAGCCACCCAAGGCACAGCTTGGAGGCTTGAACAGTGGGACATGTACA
Acknowledgments
This protocol, which was adapted from Sato et al. (2015), is partially based on the earlier works by Sato et al. (2012) and Sugiyama et al. (2006). This was supported by a grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan (Grant-in-Aid for Scientific Research [A] [25253030] to A.T., Grant-in-Aid for Scientific Research on Innovative Areas [25115502, 23112701] to A.T., Grant-in-Aid for Young Scientists [B] [25870015] to S.S.).
References
- Bartenschlager, R. and Schaller, H. (1992). Hepadnaviral assembly is initiated by polymerase binding to the encapsidation signal in the viral RNA genome. EMBO J 11(9): 3413-3420.
- Sato, S., Li, K., Kameyama, T., Hayashi, T., Ishida, Y., Murakami, S., Watanabe, T., Iijima, S., Sakurai, Y., Watashi, K., Tsutsumi, S., Sato, Y., Akita, H., Wakita, T., Rice, C. M., Harashima, H., Kohara, M., Tanaka, Y. and Takaoka, A. (2015). The RNA sensor RIG-I dually functions as an innate sensor and direct antiviral factor for hepatitis B virus. Immunity 42(1): 123-132.
- Sato, Y., Hatakeyama, H., Sakurai, Y., Hyodo, M., Akita, H. and Harashima, H. (2012). A pH-sensitive cationic lipid facilitates the delivery of liposomal siRNA and gene silencing activity in vitro and in vivo. J Control Release 163(3): 267-276.
- Sugiyama, M., Tanaka, Y., Kato, T., Orito, E., Ito, K., Acharya, S. K., Gish, R. G., Kramvis, A., Shimada, T., Izumi, N., Kaito, M., Miyakawa, Y. and Mizokami, M. (2006). Influence of hepatitis B virus genotypes on the intra- and extracellular expression of viral DNA and antigens. Hepatology 44(4): 915-924.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Sato, S., Li, K. and Takaoka, A. (2016). Infection of Human Hepatocyte-chimeric Mice with HBV and in vivo Treatment with εRNA. Bio-protocol 6(2): e1718. DOI: 10.21769/BioProtoc.1718.
Category
Microbiology > Microbe-host interactions > In vivo model > Mammal
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










