- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Cotyledon Wounding of Arabidopsis Seedlings
Published: Vol 6, Iss 2, Jan 20, 2016 DOI: 10.21769/BioProtoc.1712 Views: 12934
Reviewed by: Arsalan DaudiKaisa KajalaAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Simple Sonication Method to Isolate the Chloroplast Lumen in Arabidopsis thaliana
Jingfang Hao and Alizée Malnoë
Aug 5, 2023 2285 Views

A Plate Growth Assay to Quantify Embryonic Root Development of Zea mays
Jason T. Roberts [...] David M. Braun
Oct 20, 2023 2256 Views

Detection and Quantification of Programmed Cell Death in Chlamydomonas reinhardtii: The Example of S-Nitrosoglutathione
Lou Lambert and Antoine Danon
Aug 5, 2024 1589 Views
Abstract
Damage to plant organs through both biotic and abiotic injury is very common in nature. Arabidopsis thaliana 5-day-old (5-do) seedlings represent an excellent system in which to study plant responses to mechanical wounding, both at the site of the damage and in distal unharmed tissues. Seedlings of wild type, transgenic or mutant lines subjected to single or repetitive cotyledon wounding can be used to quantify morphological alterations (e.g., root length, Gasperini et al., 2015), analyze the dynamics of reporter genes in vivo (Larrieu et al., 2015; Gasperini et al., 2015), follow transcriptional changes by quantitative RT-PCR (Acosta et al., 2013; Gasperini et al., 2015) or examine additional aspects of the wound response with a plethora of downstream procedures. Here we illustrate how to rapidly and reliably wound cotyledons of young seedlings, and show the behavior of two promoters driving the expression of β-glucuronidase (GUS) in entire seedlings and in the primary root meristem, following single or repetitive cotyledon wounding respectively. We describe two procedures that can be easily adapted to specific experimental needs.
Keywords: PlantMaterials and Reagents
- Single wounding of seedling cotyledons
- Sterile 6 cm x 6 cm nylon mesh, 200 μm pore size (Lanz-Anliker AG, custom made)
- Standard 9 cm round Petri plates
- 3MTM MicroporeTM Tape, 1.25 cm x 9.14 m, hypoallergenic tape, standard roll (3M, catalog number: 1530-0 )
- Two 25 G x 5/8” hypodermic needles, 0.5 mm x 16 mm (BD Bioscience, catalog number: 305760 )
- Arabidopsis thaliana sterile seeds, e.g., MYC2p-GUS reporter line (Gasperini et al., 2015)
- Sterile double distilled H2O (ddH2O)
- Murashige and Skoog (MS) medium (Duchefa Biochemie, catalog number: M0221.0050 )
- MES hydrate (Sigma-Aldrich, catalog number: M8250 )
- Agar (AppliChem GmbH, catalog number: A2111 1000 )
- Standard 9 cm round Petri plates filled with 30 ml of solid half-strength Murashige and Skoog (0.5x MS) medium with 0.7% agar (see Recipes)
- Sterile 6 cm x 6 cm nylon mesh, 200 μm pore size (Lanz-Anliker AG, custom made)
- Repetitive wounding of seedling cotyledons
- 3MTM MicroporeTM Tape, 2.5 cm x 9.14 m, hypoallergenic tape, standard roll (3M, catalog number: 1530-1 )
- One 25 G x 5/8” hypodermic needle, 0.5 mm x 16 mm (BD Bioscience, catalog number: 305760 )
- One 36 gauge beveled NanoFil needle, 110 µM outer diameter (World Precision Instruments, catalog number: NF36BV )
- Standard 12 cm x 12 cm square Petri plates
- Racks for vertical growth (e.g., Milian, catalog number: 086680 )
- Arabidopsis thaliana sterile seeds, e.g., CYCB1;1p-GUS reporter line (Colón-Carmona et al., 1999)
- Sterile ddH2O
- Standard 12 cm x 12 cm square Petri plates filled with 70 ml 0.5x MS with 0.85% agar (see Recipes)
- 3MTM MicroporeTM Tape, 2.5 cm x 9.14 m, hypoallergenic tape, standard roll (3M, catalog number: 1530-1 )
- GUS staining
- Staining dish [e.g., 12 well suspension culture plate (Greiner Bio-One GmbH, Cell Star®, catalog number: 665102 )]
- Ice bucket with ice
- Microscopy slides (e.g., Thermo Fisher Scientific, Menzel-Glaser,catalog number: AD00000112E ) and coverslips (e.g., Thermo Fisher Scientific, Menzel-Glaser, catalog number: BB024060A1 )
- 90% (v/v) acetone in ddH2O
- 50 mM sodium phosphate buffer (pH 7.0)
- 70% (v/v) ethanol (EtOH) in ddH2O
- Potassium Ferrocyanide [e.g., Potassium hexacyanoferrate(II) trihydrate (Sigma-Aldrich, catalog number: 60279 )]
- Potassium Ferricyanide [e.g., Potassium hexacyanoferrate(III) (Merck Millipore Corporation, catalog number: 104973 )]
- 5-Bromo-4-chloro-3-indoxyl-beta-D-glucuronic acid (X-Gluc) (e.g., Biosynth, catalog number: B-7300 )
- Chloral hydrate (e.g., Sigma-Aldrich, catalog number: 23100 )
Safety note: acute toxicity, avoid inhalation. - Glycerol (e.g., Sigma-Aldrich, catalog number: G6279 )
- GUS staining solution (see Recipes)
- Chloride hydrate solution (see Recipes)
- Staining dish [e.g., 12 well suspension culture plate (Greiner Bio-One GmbH, Cell Star®, catalog number: 665102 )]
Equipment
- Sterile hood (laminar flow hood)
- Fine forceps style 4 (e.g., Dumont, catalog number: 0508-4-PO )
- Micropipettes (P20, P200, P1000)
- Phytotron or plant growth chamber set with the following growth conditions: 21 °C, 100 μE m-2 s-1 light intensity, 14 h light/ 10 h dark photoperiod
- Portable dissecting stereomicroscope
- Vacuum pump
- 37 °C incubator
- Stereomicroscope [e.g., Leica Microsystems, model: Leica MZ16A fitted with a camera (model: DFC310FX )]
- Differential Interference Contrast (DIC) microscope [e.g., Leica Microsystems, model: Leica DM5500 fitted with a camera (model: DFC420 )]
Procedure
- Single wounding of seedling cotyledons
Note: steps 1-3 must be performed in aseptic conditions under a sterile hood.- To equidistantly sow seeds on the media, place a printed guide underneath the petri dish (File 1A).
- Place a sterile nylon mesh on a solid 0.5x MS medium (0.7% agar) using sterilized forceps.
Note: The nylon mesh provides a solid support that allows seedlings to grow straight with a cotyledon surface that is easily accessible for wounding. - Resuspend sterile seeds in 500 µl sterile ddH2O and equidistantly plate 120 individual seeds onto the sterile mesh with a P20 micropipette set to dispense a volume of 20 µl (Figure 1A, Video 1).
Note: If more than one seed are ejected on the mesh in a single spot, the excess can be aspirated and repositioned.Video 1. Seed guide - Seal the plate with 1.25 cm wide micropore tape, stratify for 2 d in the dark at 4 °C (keep the plates horizontally, seeds facing upwards), transfer to the growth chamber in the morning and grow horizontally for 5 d.
Note: Samples can be kept in the dark by wrapping them in aluminum foil. - On the 5th day carefully open the plate (Figure 1B) and wound samples under a portable dissecting stereomicroscope. To minimize seedling movement and support the sample, place one hypodermic needle under the cotyledon with its hollowed face pointing downwards, and use the other hypodermic needle to pierce the center of the cotyledon with its sharper side (Figure 1C-D). With practice, it takes 3-4 min to wound 120 seedlings (1 plate).
- Re-seal the plate and place it back in the growth chamber. To induce the MYC2p-GUS reporter gene activity, keep seedlings in the growth chamber for 4 h prior to tissue collection. Analyze 30-60 seedlings for each treatment.
- Collect plant samples with forceps by gently lifting 5-10 seedlings at a time at the hypocotyl-cotyledon interface avoiding additional wounding or squeezing of tissues. Place them in staining dishes containing 1-2 ml 90% acetone and incubate for a minimum of 20 min on ice.
- Wash the seedlings twice with 1 ml 50 mM sodium phosphate buffer pH 7.0.
- Replace the rinsing buffer with 750 µl GUS staining solution.
- Transfer samples in a vacuum pump and vacuum-infiltrate for 5 min at room temperature, then incubate at 37 °C in the dark for 2 h.
- Stop the reaction by replacing the staining solution with 70% EtOH. Replace the 70% EtOH solution about twice a day until most tissues are no longer green (2-3 d). Samples can be stored in this solution for long periods at 4 °C if well sealed to prevent EtOH evaporation.
- Replace the 70% EtOH with chloral hydrate solution and mount samples on microscopy slides in the same chloral hydrate solution.
- Allow tissues to clarify for at least 24 h before photographing with a stereomicroscope.
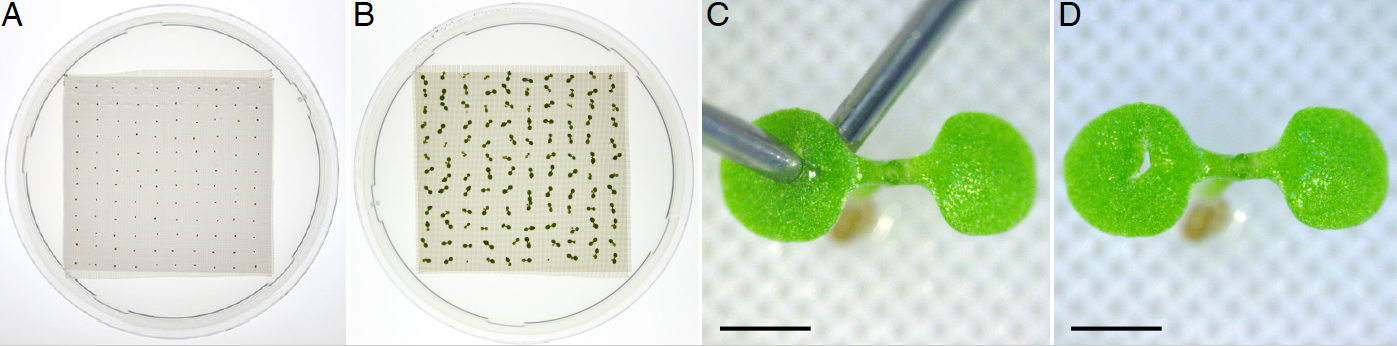
Figure 1. Single cotyledon wounding of horizontally grown 5-do Arabidopsis seedlings. Seeds are plated on media fitted with the nylon mesh (A, Video 1) and grown horizontally for 5 d after stratification (B). Seedlings are pierced with a hypodermic needle in the center of one of the cotyledons (C) resulting in a visible wound (D). Scale bars = 0.5 mm.
- To equidistantly sow seeds on the media, place a printed guide underneath the petri dish (File 1A).
- Repetitive wounding of seedling cotyledons
Note: Steps B1-4 must be performed in aseptic conditions under a sterile hood.- To equidistantly sow seeds on the media, place a printed guide underneath the petri dish (File 1B).
- Resuspend sterile seeds in 500 µl sterile ddH2O and equidistantly plate two rows of 30 seeds each on a solid 0.5x MS medium (0.85% agar) with a P20 micropipette set to dispense a volume of 20 µl (Figure 2A).
Note: If more than one seed are ejected on the mesh in a single spot, the excess can be aspirated and repositioned. - Seal the plate with 2.5 cm wide micropore tape, stratify for 2 d in the dark at 4 °C (keep the plates horizontally, seeds facing upwards), transfer to the growth chamber in the morning and grow vertically for 3 d (Figure 2B).
Note: Samples can be kept in the dark by wrapping them in aluminum foil. - In the morning (7-8 am) of the third day, carefully open the plate in aseptic conditions, place it horizontally and wound under a portable dissecting stereomicroscope. To minimize seedling movement and support the sample, place a hypodermic needle under the cotyledon with its hollowed face pointing downwards, and use the NanoFil needle to pierce the cotyledon (Figure 2C). With practice, it takes approximately 6 min to wound 60 seedlings (1 plate).
- Close the plate, return to the growth chamber and keep growing vertically.
- Repeat the wounding procedure every 12 h on alternate cotyledons, for a total of 5 wounds per seedling (Figure 2D).
- After the last 5th wound keep the seedlings in growth chamber for 2 h to induce CYCB1; 1 promoter-driven GUS reporter gene activity. Collect seedlings using forceps and gently lift them at the hypocotyl-cotyledon interface avoiding additional wounding or squeezing of tissues. Analyze 30-40 seedlings for each treatment.
- Carefully transfer 5-10 seedlings at a time into the GUS staining solution paying attention not to damage the root tips. Incubate at 37 °C in the dark for 2 h.
- Stop the reaction by replacing the GUS staining solution with 1 ml 50 mM sodium phosphate buffer pH 7.0. Samples can be stored for a few h at 4 °C but they should be imaged as soon as possible.
- Mount roots on microscopy slides in freshly prepared chloral hydrate solution and photograph with a DIC microscope.
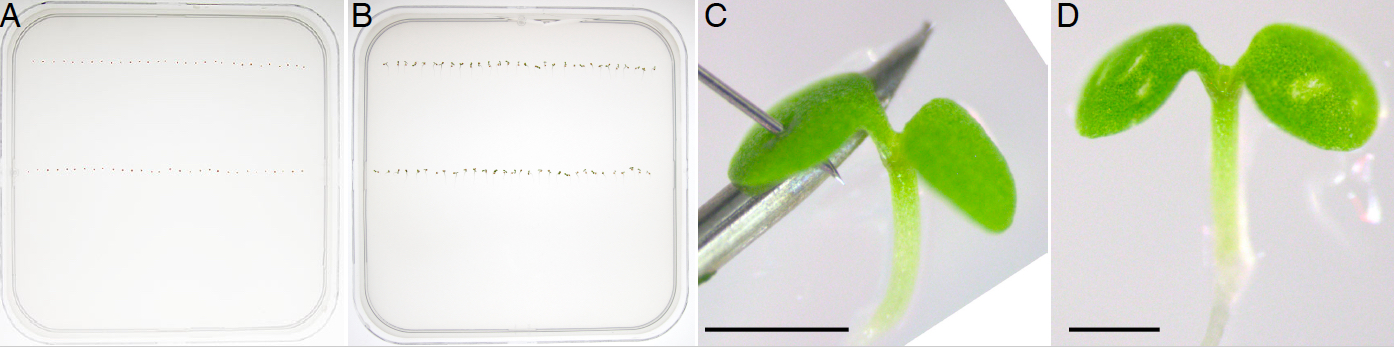
Figure 2. Repetitive cotyledon wounding of vertically grown Arabidopsis seedlings. Seeds are plated on growth media (A) and grown vertically for 3 d after stratification (B). Seedlings are pierced with a NanoFil needle on alternate cotyledons every 12 h (C) resulting in 5 visible wounds (D). Scale bars = 0.5 mm.
- To equidistantly sow seeds on the media, place a printed guide underneath the petri dish (File 1B).
Representative data
Expression of the MYC2p-GUS reporter in seedlings is normally confined to the roots, basal part of the hypocotyl and leaf primordia (Figure 3A; Gasperini et al., 2015) but single cotyledon wounding induces further activation in above ground tissues, including cotyledons and the upper hypocotyl (Figure 3B). Repetitive cotyledon wounding causes a reduction in root length due to decreased meristem cell number associated with reduced expression of the cell cycle gene CYCB1;1 (Gasperini et al., 2015), as visualized by a lower CYCB1;1p-GUS reporter activity in the root meristem of treated seedlings (Figure 3C-D).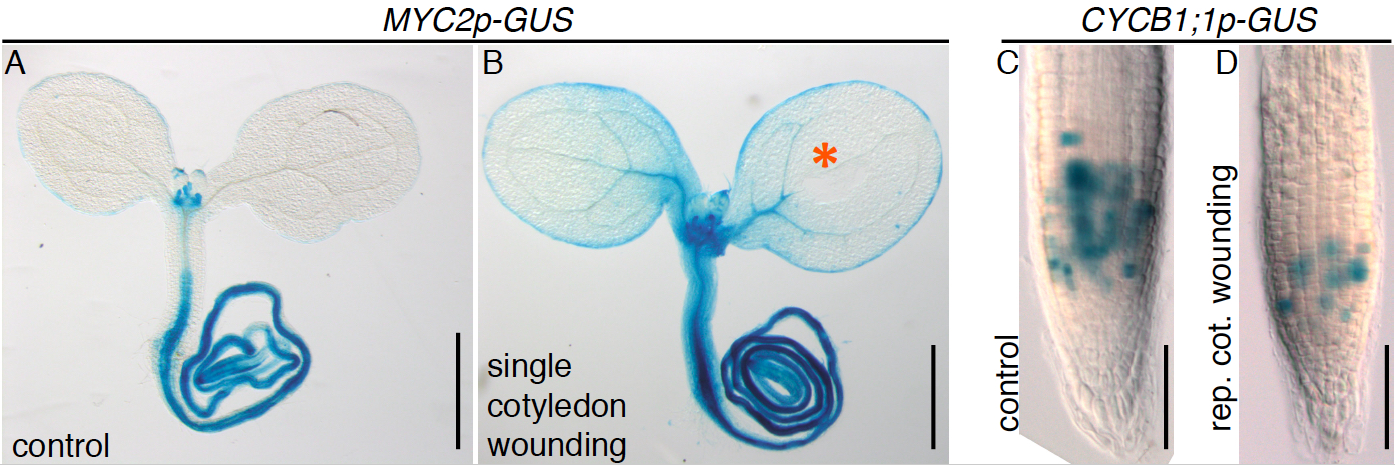
Figure 3. Examples of promoter-GUS activities following single and repetitive cotyledon wounding. MYC2p-GUS reporter activity in control (A) and single cotyledon wounded seedlings (B). The GUS enzyme converts X-Gluc into a blue colored product revealing sites of transcriptional activity. The orange asterisk indicates the wounding site. CYCB1; 1p-GUS reporter expression in the root meristem of the control (C) and seedling subjected to repetitive cotyledon wounding (D). Scale bars = 0.5 mm (A, B); 50 μm (C, D).
Notes
- The nylon mesh is sold as a large roll and should be cut into pieces of the required size. Several (8-16) mesh pieces can be wrapped in aluminum foil and sterilized together by autoclaving. The mesh can be rinsed with running tap water after use and re-sterilized multiple times.
- Any other sterile plating technique is suitable.
- Prolonged incubation of samples in chloral hydrate will improve clarifying of plant tissues that can be stored in this solution for 1-2 months. However imaging of the root tip should be done within 10-15 min after mounting in fresh chloral hydrate as meristematic cells will start to deteriorate rapidly.
- Repetitive wounding can be performed in older seedlings over longer periods of times (> 5 wounds) as long as there is sufficient tissue to wound.
Recipes
- 0.5x MS solid medium
2.15 g/L MS
0.5 g/L MES hydrate
ddH2O to final volume
Adjust pH to 5.7 with 10 M KOH
Add agar to 7 g/L (horizontal plates, 9 cm round) or 8.5 g/L (vertical plates, 12 cm square)
Autoclave
Cool down to 55-65 °C and pour into plates - GUS staining solution
Note: Prepare fresh and keep in the dark.
50 mM sodium phosphate buffer pH 7.0
10 mM EDTA pH 8.0
0.1% Triton X-100
3 mM Potassium Ferrocyanide
3 mM Potassium Ferricyanide
0.5 mg/ml 5-bromo-4-chloro-3-indolyl glucuronide (X-Gluc)
ddH2O to volume - Chloral hydrate solution
8 g chloral hydrate
2 ml glycerol
1 ml ddH2O
Acknowledgments
The single wounding protocol was developed by Acosta et al. (2013) and the repetitive wounding protocol by Gasperini et al. (2015). This work was supported by the Swiss National Science Foundation grant 31003A-138235 to E.E.F. We thank A. Chételat, A. Kurenda and C. T. Nguyen for photography and video assistance.
References
- Acosta, I. F., Gasperini, D., Chetelat, A., Stolz, S., Santuari, L. and Farmer, E. E. (2013). Role of NINJA in root jasmonate signaling. Proc Natl Acad Sci U S A 110(38): 15473-15478.
- Colon-Carmona, A., You, R., Haimovitch-Gal, T. and Doerner, P. (1999). Technical advance: spatio-temporal analysis of mitotic activity with a labile cyclin-GUS fusion protein. Plant J 20(4): 503-508.
- Gasperini, D., Chetelat, A., Acosta, I. F., Goossens, J., Pauwels, L., Goossens, A., Dreos, R., Alfonso, E. and Farmer, E. E. (2015). Multilayered organization of jasmonate signalling in the regulation of root growth. PLoS Genet 11(6): e1005300.
- Larrieu, A., Champion, A., Legrand, J., Lavenus, J., Mast, D., Brunoud, G., Oh, J., Guyomarc'h, S., Pizot, M., Farmer, E. E., Turnbull, C., Vernoux, T., Bennett, M. J. and Laplaze, L. (2015). A fluorescent hormone biosensor reveals the dynamics of jasmonate signalling in plants. Nat Commun 6: 6043.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Gasperini, D., Acosta, I. F. and Farmer, E. E. (2016). Cotyledon Wounding of Arabidopsis Seedlings. Bio-protocol 6(2): e1712. DOI: 10.21769/BioProtoc.1712.
Category
Plant Science > Plant physiology > Abiotic stress
Plant Science > Plant physiology > Tissue analysis
Plant Science > Plant immunity > Perception and signaling
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link