- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
In vitro and in vivo Limiting Dilution Assay for Colorectal Cancer
Published: Vol 5, Iss 22, Nov 20, 2015 DOI: 10.21769/BioProtoc.1659 Views: 25223
Reviewed by: HongLok LungJustine MarsolierAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Isolation and Separation of Epithelial CD34+ Cancer Stem Cells from Tgfbr2-deficient Squamous Cell Carcinoma
Heather A. McCauley and Géraldine Guasch
Sep 5, 2017 9936 Views
Abstract
The in vitro limiting dilution assay is used to determine the colorectal cancer initiating cell (CC-IC) frequency of a CC-IC enriched suspension culture, grown in growth factor enriched serum free media. The in vivo limiting dilution assay is used to determine the colorectal cancer initiating cell frequency of a primary colorectal cancer sample or an established suspension cell line using immunocompromised murine xenograft models. In vitro and in vivo limiting dilution assays (LDAs) can be used to determine the effect of a specific treatment or genetic knockdown strategy on the initiating cell frequency of a population of CC-ICs or colorectal cancer sample, respectively.
Part I. In vitro CRC LDA
Materials and Reagents
- Centrifuge tubes (BD Falcon, catalog numbers: 352096 and 352070 )
Note: Currently, it is “Corning Inc., FalconTM, catalog numbers: 352096 and 352070”. - 7x sterile, non-tissue culture 96 well U-bottom plates (Corning Inc., FalconTM, catalog number: 351177 )
- FACS tubes with 0.35 μm filter (BD Falcon, catalog number: 352235 )
Note: Currently, it is “Corning Inc., FalconTM, catalog number: 352235”. - Wash media (DMEM/F-12) (Thermo Fisher Scientific, GibcoTM, catalog number: 11320-033 )
- 0.4% Trypan blue solution (Life Technologies, catalog number: 15250-061 )
Note: Currently, it is “Thermo Fisher Scientific, GibcoTM, catalog number: 15250-061”. - Permanent marker (VWR International, catalog number: 52877-310 )
- 0.25% Trypsin (Life Technologies, catalog number: 25200-056 )
Note: Currently, it is “Thermo Fisher Scientific, GibcoTM, catalog number: 25200-056”. - Penicillin-streptomycin-amphotericin B (1%) (Life Technologies, catalog number: 15240-062 )
Note: Currently, it is “Antibiotic-Antimycotic (100x) (Thermo Fisher Scientific, GibcoTM, catalog number: 15240-062)". - L-glutamine (2 mM) (Invitrogen, catalog number: 25030081 )
Note: Currently, it is “Thermo Fisher Scientific, GibcoTM, catalog number: 25030081”. - Non-essential amino acids (1x) (Thermo Fisher Scientific, HycloneTM, catalog number: SH3023801 )
- Sodium pyruvate (1 mM) (Thermo Fisher Scientific, HycloneTM, catalog number: SH3023901 )
- HEPES (1x) (Invitrogen, catalog number: 15630080 )
Note: Currently, it is “Thermo Fisher Scientific, GibcoTM, catalog number: 15630080”. - Heparin sodium salt (4 μg/ml) from porcine intestinal mucosa (Sigma-Aldrich, catalog number: H3149-100KU )
- Lipids (Sigma-Aldrich, catalog number: L0288 )
- Human Epidermal Growth Factor Recombinant (EGF) (20 ng/ml) (Biomartcanada, catalog number: RKP01133 )
- Human basic Fibroblast Growth Factor Recombinant (bFGF) (10 ng/ml) (Biomartcanada, catalog number: RKP09038 )
- N2 supplement-A (STEMCELL Technologies, catalog number: 07152 )
- NeuroCult SM1 Neuronal (STEMCELL Technologies, catalog number: 05711 )
- Sytox blue (Life Technologies, catalog number: S11348 )
Note: Currently, it is “Thermo Fisher Scientific, Molecular ProbesTM, catalog number: S11348”. - Phosphate-buffered saline (PBS) (Life Technologies, catalog number: 10010023 )
Note: Currently, it is “Thermo Fisher Scientific, GibcoTM, catalog number: 10010023”. - 2% fetal bovine serum (FBS) (Life Technologies, catalog number: 26140-111 )
Note: Currently, it is “Thermo Fisher Scientific, Gibco™, catalog number: 26140-111 ”. - Serum free cell culture media (see Recipes)
Equipment
- 37 °C Incubator
- Light microscope (Leica Microsystems, model: DM IL LED )
- Hemocytometer (Hausser Scientific, catalog number: 1483 )
- Pipette aid (INTEGRA Biosciences AG, catalog number: 155 000 )
- Pipettes (BD Falcon, catalog number: 357530 )
Note: Currently, it is “Corning Inc., FalconTM, catalog number: 357530”. - Flow cytometry assisted cell sorter (FACS)
Procedure
Note: The range of cell concentrations used will depend on the frequency of initiating cells in your fraction. An initial LDA using a wide range of concentrations can be used to find the best range of cell doses, ideally it should include doses with 100% sphere formation down to cell doses with no sphere formation, as well as, a range of doses in between. For an LDA to be statistically valid one must first test whether their sample complies with the Poisson single-hit model, which assumes that the number of biologically active units in a culture varies according to Poisson distribution. For additional information and calculations please refer to Extreme Limiting Dilution Analysis (ELDA) site (http://bioinf.wehi.edu.au/software/elda/), and the accompanying paper (Hu and Smyt, 2009). Ex: if the initiating frequency is approximately (1:50-1:200), then the range indicated in Table 1 will be sufficient to obtain statistically significant results.
- Seeding for an in vitro LDA
- Grow cells to confluence in a T175 flask at 37 °C.
- Centrifuge cells (300-450 x g) for 5 min at room temperature, then remove supernatant.
- Resuspend cell pellet in ~3 ml of trypsin and incubate at 37 °C for the necessary time to obtain single cells.
- Remove cells from incubator and resuspend with ~10 ml of wash media to stop trypsin reaction.
- Centrifuge cells (300-450 x g) for 5 min at room temperature, then remove supernatant.
- Completely resuspend pellet in enough cell media so that you will have a final cell concentration of about 1 x 106 cells/ml in order to make cell counting on the hemocytometer easier for you.
- Mix 10 μl of cell mixture with 10 μl of trypan blue solution and load mixture onto a hemocytometer to count the number of live cells using a light microscope. Be sure to count cells in all four coordinates and divide by 2 to obtain the concentration of cells in your solution (__x 104/ml). Please refer to http://www.abcam.com/protocols/counting-cells-using-a-haemocytometer for a further explanation on how to use a hemocytometer.
- Seed cells either manually or by using a FACS machine.
Manual cell seeding- Serially dilute cells to obtain the following final cell concentrations
- Tube 1 (1,000 cells/well): 27,000 cells in 5.4 ml media
- Tube 2 (100 cells/well): 10,000 cells in 20 ml media
- Tube 3 (10 cells/well): 2,000 cells in 40 ml media
- Tube 4 (1 cell/well): 300 cells in 60 ml media
- Tube 1 (1,000 cells/well): 27,000 cells in 5.4 ml media
- Thoroughly mix cell solutions each time, before dispensing 200 μl of each into sterile non-tissue culture 96 well U-bottom plates according to Table 1.
- Centrifuge plates containing single cells (300-450 x g) for 5 min at room temperature, and using a microscope, determine which wells are confirmed to have only one cell. Using a marker, circle those wells containing single cells.
- Return plates to 37 °C incubator as soon as possible.
Table 1. Cell seeding for an in vitro limiting dilution assayCell concentration (#cells/well) Number of 96 well plates Number of wells 1,000 0.25 24 100 1 96 10 2 192 1 3 288
- Remove ~2-3 x 106 cells from your cell solution and put it into a centrifuge.
- Centrifuge cells (300-450 x g) for 5 min at room temperature.
- While cells are spinning, mix sytox blue with PBS + 2% FBS to obtain a final working concentration of 10 nM-1 μM depending on your cell line (please refer to product manual for further information). Avoid exposure of sytox blue solution to light.
- Remove supernatant and completely resuspend pellet in 2-3 ml sytox blue working solution.
- Filter cell/sytox blue solution through a 0.35 μm filter to ensure a single cell suspension before putting cells into a FACS tube.
- Keep the cell solution on ice and in the dark.
- Add 200 μl of cell media to 7 sterile non-tissue culture 96 well U-bottom plates.
- Use a FACS machine to sort cells into 96 well plates containing cell media, gating out the dead cells that are positive for sytox blue. Sytox blue has an excitation of 444 nm and an emission of 480 nm (please refer to product manual for further information on how to read sytox blue on a FACS machine).
- Centrifuge plates containing single cells (300-450 x g) for 5 min at room temperature, and using a microscope, determine which wells are confirmed to have only one cell. Using a marker, circle those wells containing single cells.
- Return plates to 37 °C incubator as soon as possible.
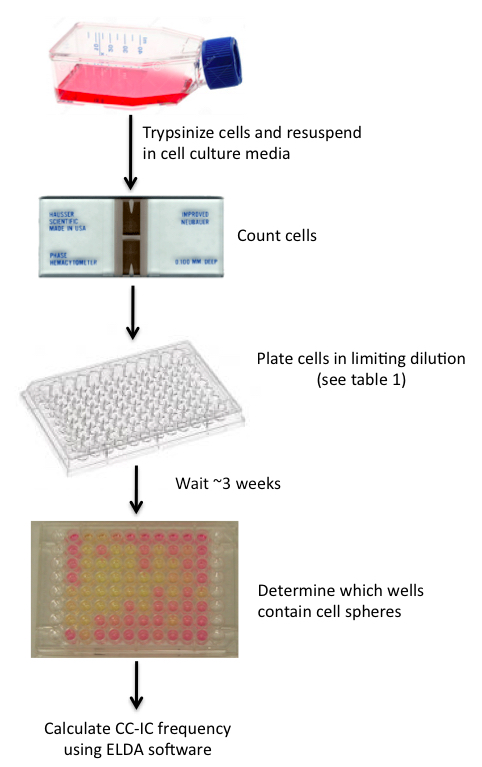
Figure 1. In vitro LDA schematic
- Serially dilute cells to obtain the following final cell concentrations
- Grow cells to confluence in a T175 flask at 37 °C.
- Scoring an in vitro LDA
- After ~2-4 weeks remove the plates from the incubator and using a microscope, determine which wells contain cell spheres. For the 1 cell/well plates, be sure to only score those wells that were originally confirmed to contain a single cell.
- Use ELDA: Extreme Limiting Dilution Analysis online software to calculate the cancer cell initiating frequency and significance (http://bioinf.wehi.edu.au/software/elda/).
- After ~2-4 weeks remove the plates from the incubator and using a microscope, determine which wells contain cell spheres. For the 1 cell/well plates, be sure to only score those wells that were originally confirmed to contain a single cell.
Part II. In vivo LDA
Materials and Reagents
- Centrifuge tubes (BD Falcon, catalog numbers: 352096 and 352070 )
Note: Currently, it is “Corning Inc., FalconTM, catalog numbers: 352096 and 352070”. - 1 ml syringe without needle (Restek Corporation, catalog number: 22766 )
- 100 x 1 ml syringes with (281/2G) needles (BD, catalog number: 329420 )
- 50x NOD-SCID or NSG mice
- ~5 ml matrigel (Corning, catalog number: 354248 )
- Wash media (DMEM/F-12) (Thermo Fisher Scientific, GibcoTM, catalog number: 11320-033 )
- 0.4% Trypan blue solution (Life Technologies, catalog number: 15250-061 )
Note: Currently, it is “Thermo Fisher Scientific, GibcoTM, catalog number: 15250-061”. - 0.25% Trypsin (Life Technologies, catalog number: 25200-056 )
Note: Currently, it is “Thermo Fisher Scientific, GibcoTM, catalog number: 25200-056”. - Penicillin-streptomycin-amphotericin B (1%) (Life Technologies, catalog number: 15240-062 )
Note: Currently, it is “Antibiotic-Antimycotic (100x) (Thermo Fisher Scientific, GibcoTM, catalog number: 15240-062)". - L-glutamine (2 mM) (Invitrogen, catalog number: 25030081 )
Note: Currently, it is “Thermo Fisher Scientific, GibcoTM, catalog number: 25030081”. - Non-essential amino acids (1x) (Thermo Fisher Scientific, HycloneTM, catalog number: SH3023801 )
- Sodium pyruvate (1 mM) (Thermo Fisher Scientific, HycloneTM, catalog number: SH3023901 )
- HEPES (1x) (Invitrogen, catalog number: 15630080 )
Note: Currently, it is “Thermo Fisher Scientific, GibcoTM, catalog number: 15630080”. - Heparin sodium salt (4 μg/ml) from porcine intestinal mucosa (Sigma-Aldrich, catalog number: H3149-100KU )
- Lipids (Sigma-Aldrich, catalog number: L0288 )
- Human Epidermal Growth Factor Recombinant (EGF) (20 ng/ml) (Biomartcanada, catalog number: RKP01133 )
- Human basic Fibroblast Growth Factor Recombinant (bFGF) (10 ng/ml) (Biomartcanada, catalog number: RKP09038 )
- N2 supplement-A (STEMCELL Technologies, catalog number: 07152 )
- NeuroCult SM1 Neuronal (STEMCELL Technologies, catalog number: 05711 )
- Serum free cell culture media (see Recipes)
Equipment
- 37 °C Incubator
- Light microscope (Leica Microsystems, model: DM IL LED )
- Hemocytometer (Hausser Scientific, catalog number: 1483 )
- Pipette aid (INTEGRA Biosciences AG, catalog number: 155000 )
- Pipettes (BD Falcon, catalog number: 357530 )
Note: Currently, it is “Corning Inc., FalconTM, catalog number: 357530”. - Mouse ear punch (Kent Scientific Corporation, catalog number: INS750075-5 )
Procedure
Note: The range of cell concentrations used will depend on the frequency of initiating cells in your fraction. An initial LDA using a wide range of concentrations can be used to find the best range of cell doses, ideally it should include doses with 100% tumor formation down to cell doses with no tumor formation, as well as, a range of doses in between. For an LDA to be statistically valid one must first test whether their sample complies with the Poisson single-hit model, which assumes that the number of biologically active units in a culture varies according to Poisson distribution. For additional information and calculations please refer to ELDA site (http://bioinf.wehi.edu.au/software/elda/), and the accompanying paper (Hu and Smyt, 2009). Ex: if the initiating frequency is approximately (1:1,000-1:5,000), then the range indicated in Table 2 will be sufficient to obtain statistically significant results.
- Preparing cell syringes
- Grow cells to confluence in a T175 flask at 37 °C.
- Centrifuge cells (300-450 x g) for 5 min at room temperature, then remove supernatant.
- While cells are spinning, thaw matrigel on ice.
- Resuspend pellet in ~3 ml of trypsin and incubate at 37 °C for the necessary time to obtain single cells.
- Remove cells from incubator and resuspend with ~10 ml of wash media to stop trypsin reaction.
- Centrifuge cells (300-450 x g) for 5 min at room temperature, then remove supernatant
- Completely resuspend pellet in enough cell media so that you will have a final cell concentration of about 3 x 106 cells/ml in order to make cell counting on the hemocytometer easier for you. Ensure that your cell concentration is no less than 2 x 106 cells/ml to allow for the cell concentrations listed in step A9.
- Mix 10 μl of cell mixture with 10 μl of trypan blue and load mixture onto a hemocytometer to count the number of live cells using a light microscope. Be sure to count cells in all four coordinates and divide by 2 to obtain the concentration of cells in your solution (__ x 104/ml). Please refer to http://www.abcam.com/protocols/counting-cells-using-a-haemocytometer for a further explanation on how to use a hemocytometer.
- Serially dilute cells so that you have the following final cell concentrations.
- Tube 1 (50,000 cells/injection): 600,000 cells in 360 μl media
- Tube 2 (10,000 cells/injection): 120,000 cells in 360 μl media
- Tube 3 (1,000 cells/ injection): 12,000 cells in 360 μl media
- Tube 4 (100 cells/injection): 1200 cells in 360 μl media
- Tube 5 (10 cells/injection): 120 cells in 360 μl media
- Tube 1 (50,000 cells/injection): 600,000 cells in 360 μl media
- Prepare syringes (with needles) by removing the plunger and adding necessary labels (i.e., cell concentration, treatment etc.).
- Using a syringe (without a needle), aspirate the matrigel and deposit a small drop of gel (~200 μl) into the base of syringe.
- Thoroughly mix the desired cell solution before adding 30 μl to the droplet of matrigel.
- Carefully reinsert the plunger, making sure not to expel the matrigel solution out of the syringe.
- Keep the loaded syringes on ice until ready to use.
Table 2. Cell concentrations and injections for an in vivo limiting dilution assayCell concentration
(#cells/injection)Number of injections
(2 injections/mouse)Number of mice 50,000 20 10 10,000 20 10 1,000 20 10 100 20 10 10 20 10 Total: 100 50 - Repeat this until you have 100 syringes (20 syringes per cell concentration), as shown in Table 2.
- Grow cells to confluence in a T175 flask at 37 °C.
- Injecting mice
- Lightly sedate mice with isoflurane and ear notch them to identify each mouse (see Laboratory Animal Medicine, 2015, for more information on animal identification).
- Inject mice subcutaneously with cells on both the right and left flank, using a new syringe for each injection (see Figure 2). Each mouse should have two injections, one on each flank (see Table 2).
- Return mice to their cage to recover.
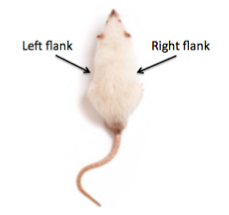
Figure 2. Subcutaneous injection sites on the right and left flank of a mouse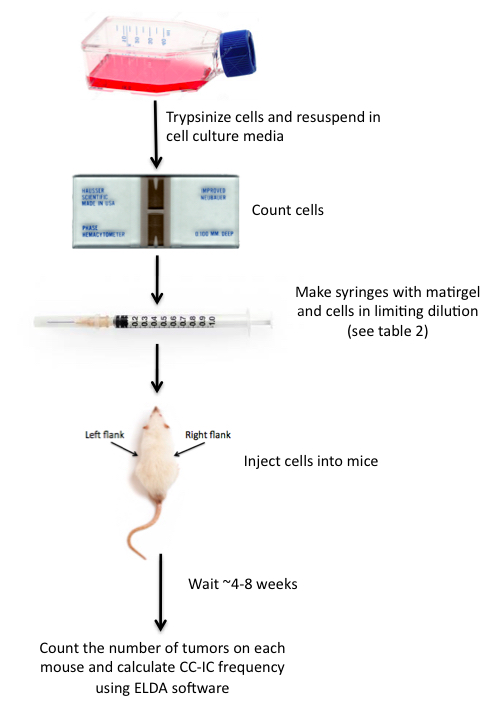
Figure 3. In vivo LDA schematic
- Lightly sedate mice with isoflurane and ear notch them to identify each mouse (see Laboratory Animal Medicine, 2015, for more information on animal identification).
- Scoring an in vivo LDA
- After ~4-8 weeks, determine which mice have developed tumors.
- Continue to monitor mice with 0-1 tumors for up to 6 months, or as long as ethically permitted, to ensure there is no late tumor formation.
- Use ELDA: Extreme Limiting Dilution Analysis online software to calculate the cancer cell initiating frequency and significance (http://bioinf.wehi.edu.au/software/elda/).
- After ~4-8 weeks, determine which mice have developed tumors.
Notes
- Results will vary slightly when repeating an LDA, however when comparing treated groups, the LDA results should convey the same overall result each time. For example; treatment ‘A’ always produces a lower, statistically significant, initiating frequency compared to a control treatment ‘B’.
- Increasing the number of replicates in both in vitro and in vivo LDAs will increase the preciseness of the assay.
Recipes
- Serum free cell culture medium
DMEM/F-12 (1:1 ratio) supplemented with:- Penicillin-streptomycin-amphotericin B (1%) 5 ml/500 ml
- L-glutamine (2 mM) 5 ml/500 ml
- Non-essential amino acids (1x) 5 ml/500 ml
- Sodium pyruvate (1 mM) 5 ml/500 ml
- HEPES (1x) 5 ml/500 ml
- Heparin sodium salt (4 μg/ml) from porcine intestinal mucosa
- Lipids 1 ml/500 ml
- Human Epidermal Growth Factor Recombinant (EGF) (20 ng/ml)
- Human basic Fibroblast Growth Factor Recombinant (bFGF) (10 ng/ml)
- N2 supplement-A 5 ml/500 ml
- NeuroCult SM1 Neuronal 2 ml/500 ml
- Penicillin-streptomycin-amphotericin B (1%) 5 ml/500 ml
References
- Counting cells using a hemocytometer: Abcam.
- Fazekas de St, G. (1982). The evaluation of limiting dilution assays. J Immunol Methods 49(2): R11-23.
- Hu, Y. and Smyth, G. K. (2009). ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J Immunol Methods 347(1-2): 70-78.
- Kreso, A., van Galen, P., Pedley, N. M., Lima-Fernandes, E., Frelin, C., Davis, T., Cao, L., Baiazitov, R., Du, W., Sydorenko, N., Moon, Y. C., Gibson, L., Wang, Y., Leung, C., Iscove, N. N., Arrowsmith, C. H., Szentgyorgyi, E., Gallinger, S., Dick, J. E. and O'Brien, C. A. (2014). Self-renewal as a therapeutic target in human colorectal cancer. Nat Med 20(1): 29-36.
- Strijbosch, L. W., Buurman, W. A., Does, R. J., Zinken, P. H. and Groenewegen, G. (1987). Limiting dilution assays. Experimental design and statistical analysis. J Immunol Methods 97(1): 133-140.
- Talcott, M., Akers, W., and Marini, R. (2015). Techniques of experimentation. In: Anderson, L. and Otto, G. (eds). Laboratory Animal Medicine. Elsevier, 1202-1204.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Agro, L. and O’Brien, C. A. (2015). In vitro and in vivo Limiting Dilution Assay for Colorectal Cancer. Bio-protocol 5(22): e1659. DOI: 10.21769/BioProtoc.1659.
Category
Cancer Biology > Cancer stem cell > Tumor formation > Self-renewal
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link