- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Quantifying the Permeability of the Apoplastic Water Barrier in Cosmos Petals
Published: Vol 5, Iss 22, Nov 20, 2015 DOI: 10.21769/BioProtoc.1652 Views: 7629
Reviewed by: Tie LiuArsalan DaudiAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Simple Sonication Method to Isolate the Chloroplast Lumen in Arabidopsis thaliana
Jingfang Hao and Alizée Malnoë
Aug 5, 2023 2285 Views

A Plate Growth Assay to Quantify Embryonic Root Development of Zea mays
Jason T. Roberts [...] David M. Braun
Oct 20, 2023 2256 Views

Detection and Quantification of Programmed Cell Death in Chlamydomonas reinhardtii: The Example of S-Nitrosoglutathione
Lou Lambert and Antoine Danon
Aug 5, 2024 1589 Views
Abstract
The capacity of plants to minimize uncontrolled water loss is essential for survival in adverse and changing climatic conditions. In order to assess and compare the effectiveness of apoplastic barriers to water, the permeability of the barrier must first be quantified. Studies have accomplished this directly by quantifying tritium flux or indirectly by measuring the influx/efflux of water surrogates such as dyes, chlorophyll, and herbicides. Other studies have relied on comparative methods such as survival rates after drought. These methods rely on radioactive material, correlations, or qualitative comparisons. However, a quantitative method is necessary that directly measures water efflux and that allows easy comparisons within and between experiments, plant parts, plant species, and especially research laboratories. Here we outline in detail a gravimetric protocol first described by Schönherr and Lendzian (1981) that can be set up in less than half a day and completed in one to ten days depending on the plant barrier. This approach has been used in numerous studies on leaf and fruit cuticles and recently also on petals from cosmos (Cosmos bipinnatus; Buschhaus et al., 2015).
Materials and Reagents
- Leaf punch (12 millimeter diameter)
- Syringe (10 ml)
- Syringe (1 ml) with needle
- Pipette tip (100 µl)
- Pencil (unused)
- Sand paper (80 grit) (other grits may work but the author has not tested them)
- Tape (3M, Scotch®)
- Silica desiccant (Sorbsil Chameleon, BDH Prolabo)
- Plant material with minimum dimensions of 13 x 13 millimeters (e.g., ray petals 3-days post-anthesis from Cosmos bipinnatus cv. sensation pinkie; Seeds were obtained from Stokes Seeds)
- Silicon grease (Dow Corning Corporation, catalog number: Z273554 )
- De-ionized water
- Fertilizer (e.g., MiracleGro)
Equipment
- Cylinders (machined in-house with an 11 mm diameter opening and an internal capacity of at least 1 ml; Figure 1. Further details are provided in the ‘Notes’ section)
- Metal meshing (10 cm x 20 cm x 0.2 cm; 0.7 cm hole diameter; 0.5 holes cm-2)
- Plastic box with tight fitting lid (10 cm x 20 cm)
- Incubator (Thermo Fisher Scientific, model: Isotemp 210 )
- Balance (Sartorius AG, model: CP1245 )
- Computer
Software
- Spreadsheet software (e.g., Microsoft Excel)
- Data logger program to transfer balance data directly to computer (optional)
Procedure
- Set an incubator to 25 °C. Allow sufficient time (e.g., overnight in our case) for the temperature to stabilize before plant material is harvested.
- Warm n+1 ml of water to 25 °C, where n equals the number of samples to be analyzed. We place a bottle of water in the incubator when we first turn it on.
- Fill the plastic container to a depth of approximately 1 cm with dry desiccant (Figure 1). Place the metal meshing on top of the desiccant to provide a solid base for the cylinders. Put the lid on the plastic container and then pre-warm to 25 °C in the incubator. We warm the container overnight just like the water in step 2, but one hour is likely sufficient. If the incubator does not occlude light, ensure that the selected plastic container does not transmit light.
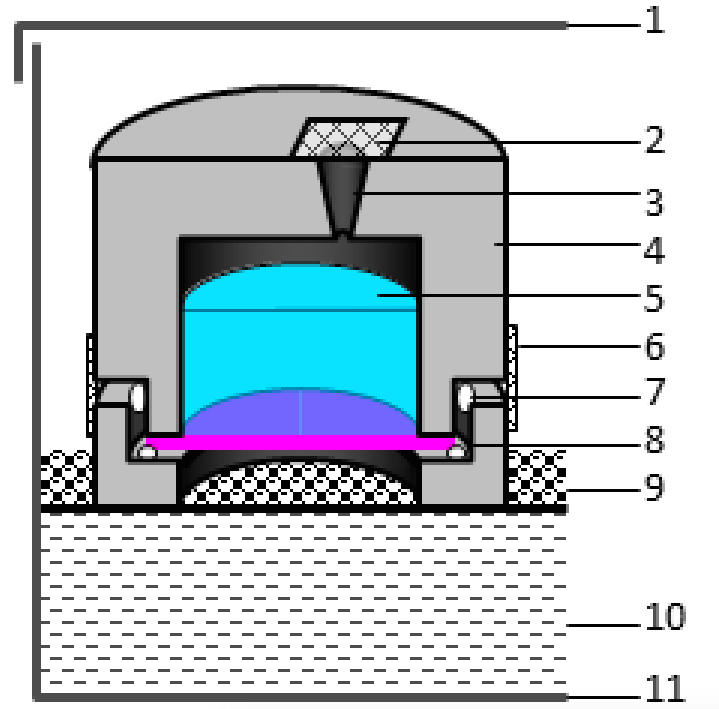
Figure 1. Cross-sectional diagram of the experimental set-up. Desiccant (10) is layered in to the bottom of a plastic container (11) that has a tight-fitting lid (1). A firm mesh surface (9) provides support for the cylinder (4). A petal disc (8) is sealed with silicon grease (7) in the 1.1 cm opening of the cylinder. Tape (6) provides an additional seal and holds the two parts of the cylinder together. The central opening in the chamber (5) is filled with water through a tiny hole (3) before the hole is sealed with tape (2). - Assemble a ‘sealant applicator’ (Figure 2A). Screw a 100 µl pipette tip into the end of a plastic 10 ml syringe. Cut the end of the pipette tip to the desired diameter, which in our case is 1.5-2 mm. Remove the plunger and fill the syringe with a sealant such as high temperature vacuum grease. Re-insert the plunger. Slowly pressing the plunger allows for a thin bead of sealant to be evenly and continuously applied to the chambers. Other sealants such as Vaseline are likely sufficient; the authors however have not experimented with this.
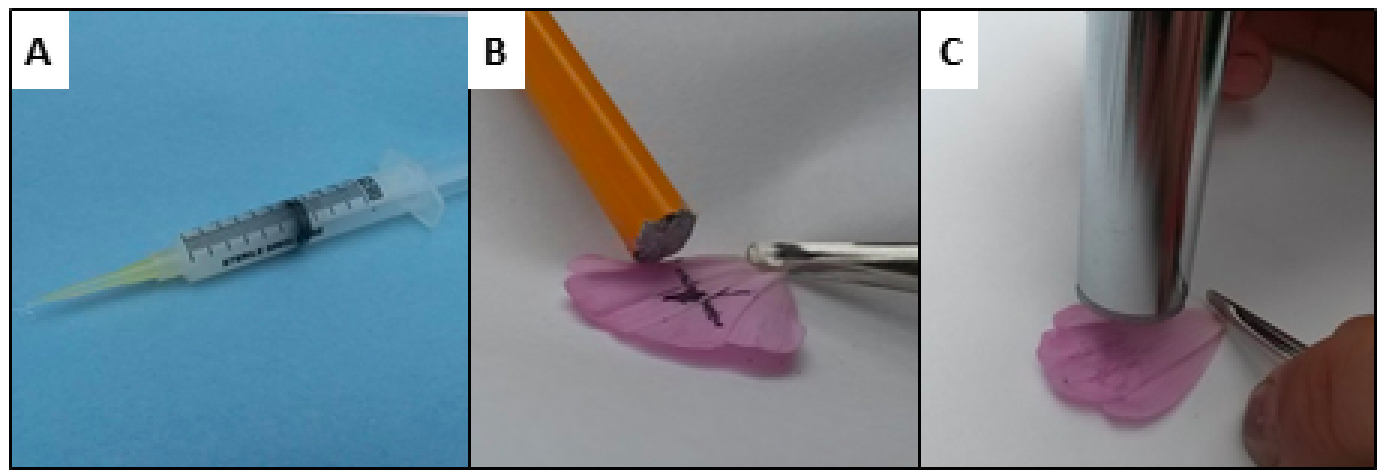
Figure 2. Tools used in this protocol. A sealant applicator was constructed by screwing a 100 µl pipette tip into the end of a disposable syringe (A). The syringe is then filled with sealant. Since the pipette tip is tapered, the diameter of the applied sealant bead can be modified by trimming the pipette tip. A surface abrader was constructed by gluing a small disc of 80 grit sand paper to the bottom of a pencil (B). A 12 mm or 13 mm leaf punch was used to cut a disc from the petal (C). - Prepare the cylinders by applying a bead of sealant (silicon grease) using the ‘sealant applicator’ in a continuous, single ring on the inner surface of the cylinder lid and in a continuous, single ring on the cylinder bottom where it will contact the lid (Figure 4A). The sealant will be spread out later (steps 10-11) in the process of joining together the two surfaces to be sealed. Pre-warm the cylinders to 25 °C by putting them in the incubator.
- Construct a ‘surface abrader’ (Figure 2B). Glue a circle of sand paper on to the eraser end of a new pencil. Try using a hole punch to obtain a suitably sized circle of sand paper.
- Obtain plant material - cosmos (Cosmos bipinnatus cv. Sensation pinkie) ray petals from flowers 3-7 days post anthesis - with dimensions greater than 13 mm by 13 mm (Figure 3A). Controlled conditions of plant growth aid in reproducibility. Astomatous leaves of other plants may also be used, provided that they have an area greater than 13 mm by 13 mm. If the adaxial and abaxial surfaces are indistinguishable, mark the opposing surface with a permanent marker (Figure 3B); do not mark the surface of interest.
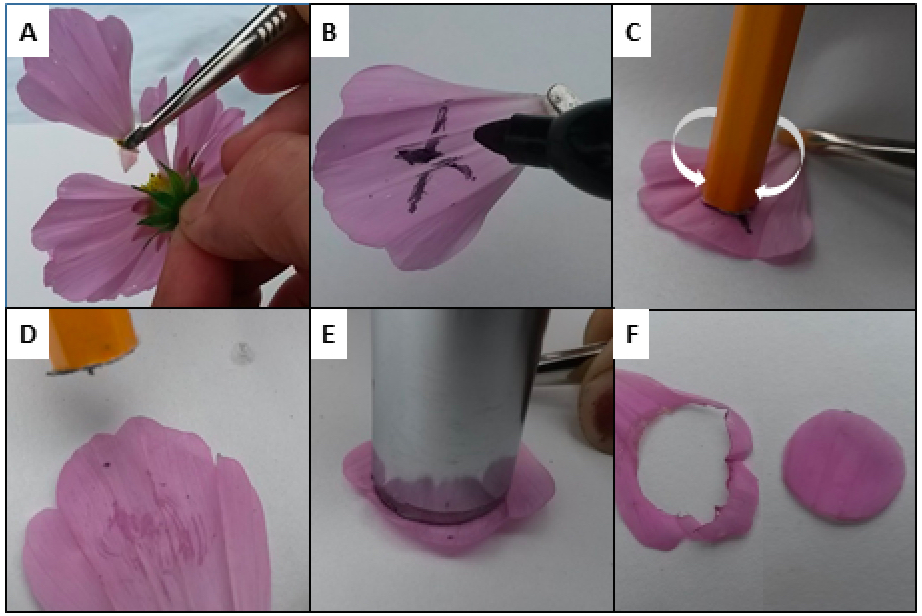
Figure 3. Preparation of petal disc for water permeance analysis. A ray petal is harvested from a flower three days post anthesis (A). The side opposite from the surface-of-interest is marked in situations where the adaxial surface is indistinguishable from abaxial surface (B). The surface abrader is gently twirled on this opposite surface (C) to scratch through the surface such that water can more easily penetrate from this side (D). A cork borer is used to cut the petal (E) into a disc, ready for sealing into the cylinder (F). - Gently abrade the opposing, marked surface (not the surface of interest) with a gentle rotation of the sandpaper-end of the ‘surface abrader’ (Figure 3C-D). The purpose of this step is to facilitate water ingress, thereby ensuring a maximal internal water concentration. Failure to disturb the cuticular barrier on this surface will invalidate the water loss measurements. A razor blade may be used to cut slits instead of abrasion with the sandpaper. However, this requires steady hands and/or thick plant material to prevent slicing through the cuticle of interest.
- Using the leaf punch (cork borer; Figure 2C), stamp a disc of plant tissue measuring 12 mm in diameter (Figure 3E-F).
- Place the plant disc into the lid such that the surface-of-interest faces the out towards the desiccant (Figure 4B-C). Use the ‘surface abrader’ to gently tap around the disc circumference such that the disc makes continuous contact with the sealant bead.
- Gently slide the chamber bottom onto the lid. Rotate the bottom a quarter turn to ensure the sealant is evenly spread between the cylinder lid and bottom.
- Wrap a strip of tape around the cylinder such that it overlaps both the lid and the bottom. This provides a secondary seal as well as prevents the lid and bottom from separating in subsequent handling (Figure 4D-E).
- Fill the cylinder with 1 ml of the pre-warmed water through the small opening in the bottom of the cylinder (Figure 1 Item 3). We used a syringe to facilitate this transfer. However, do not insert the syringe needle so far into the hole that it punctures the plant disc. Also, gently expel the water so as not to disturb the sealed petal disc. Seal the water-filling hole with a piece of clear tape.
- Label the cylinder. We number (or letter) our cylinders and record this number with a description of the samples, including plant species, plant part, surface, age of specimen, treatment, and date.
- Place the cylinder in the plastic container and return to the incubator.
- Repeat steps 8-15 for each sample to be analyzed.
- Leave the cylinders in the desiccant container in the 25 °C incubator overnight to stabilize.
- Weigh the cylinders, recording the time of measurement and mass, and return them to the desiccant container and incubator. Although optional, connecting the balance to a computer via a data logging program to record the time and mass in a Microsoft Excel-compatible file format simplifies data handling. It is assumed that prior to weighing the cylinders the balance has been calibrated according to the manufacturer’s instructions. We recommend also weighing a standard of similar mass to the samples. This allows the accuracy of the balance to be confirmed when the measurements are made over several days (see following step).
- Repeat step 18 at set time intervals. The elapsed time must allow sufficient water loss such that the new mass decreases from the previous mass by more than the error of the balance. For fairly permeable plant surfaces, e.g., cosmos petals (Buschhaus et al., 2015), a measurement every 2 h is adequate since, for our balance, d = 0.1 mg. However, samples are typically weighed once per day to decrease the percent error. More water-resistant plant surfaces may require even longer intervals than this. The time interval may be increased or decreased to account for the precision of your balance.
While each cylinder is removed for weighing, inspect the colour of the desiccant underneath. If the desiccant immediately under the cylinder has changed to the hydrated colour, the plant disc has likely ruptured. Gently turn the cylinder over and inspect the plant disc for macroscopic tears and discard torn samples. - Plot the cumulative change in mass per cumulative change in time. The linear regression line typically accounts for greater than 97% of the observed variation. A quick examination of the plot when r2 values are less than 0.97 usually reveals a distinct bend in the line at a point where either the plant disc ruptured or the chamber ran out of water. Representative data can be found in Figure 2 of Buschhaus et al. (2015).
- Calculate the permeance P (m s-1) according to the equation P = F A-1 C-1. F is the flow (mg s-1) and equals the slope of the linear regression line from the previous step. A is the exposed area of the leaf disc. Ensure that this is the true surface area and not just the apparent surface area. This is especially important for the adaxial surface of petals where the epidermal cells frequently are not flat. C is the change in water vapour concentration (23.07 mg m-3 at 25 °C). Convention dictates that the geometric mean is reported as the data are typically skewed towards higher permeances (Baur, 2008). For a further explanation of the equation, see Schreiber and Schönherr (2009).
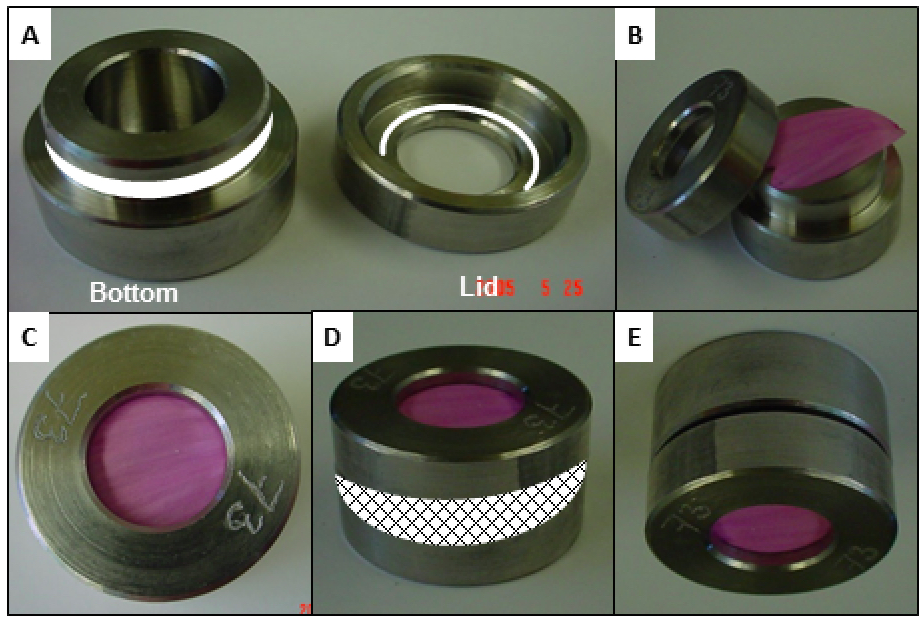
Figure 4. Cylinder for the gravimetric measurement of water permeance. Silicon grease (white bands) is applied in a ring on the lid and on the bottom of the cylinder (A). The petal disc is placed between the openings in the lid and bottom (B) and the lid is pressed firmly onto the bottom (C). The lid and bottom are secured together with a strip of tape (hatched white; D) to complete the cylinder assembly (E).
Notes
- The dimensions and composition of the cylinder are adaptable. For ease of subsequent calculations, the opening into the center of the cylinder should have a constant diameter. We find that a diameter of 11 mm was satisfactory for Cosmos petals and also allowed the cylinders to be used for the analysis of leaves from other species. The internal volume should hold at least 1 ml of water as this permits measurable water loss (up to a 1 g change-in-mass for a balance that reads to 0.0001 g) before running out. A 2 mm diameter hole was drilled in the bottom to allow the cylinder to be filled with water after the petal was sealed across the opening. In our case, the cylinders were machined out of stainless steel. However, any material not permeable to water should be adequate. External dimensions were approximately 2.5 cm by 2 cm (diameter by height).
- In order to test your sealing technique, seal a penny across the opening of the cylinder instead of the plant disc. With this set-up, no change in mass should be observed.
- Although not an issue for petals (or astomatous leaves), many leaves have stomates that provide an alternative route for water movement through the barrier. The cylinders may be incubated under conditions that promote stomate closure but care must still be exercised in interpreting the results.
- Several variations can be explored with this experimental set-up. After quantifying the barrier in a plant disc, the epicuticular wax layer can be removed and the disc re-analyzed. Following this, all of the cuticular wax can be removed and the disc re-analyzed. In this way the relative contributions of the cuticular wax layers can be determined.
- Easiest plant growth occurs with direct seeding of Cosmos on to soil (e.g., Sunshine mix 4; 1 plant per 15 cm diameter pot), weekly watering with fertilizer, and growth conditions of constant 20 °C temperature under 12 h day (approx. 150 µmol m-2 s-1 photosynthetically active radiation)/12 night conditions. If space is limited, seeds can first be sprouted prior to transplanting to soil as documented in Buschhaus et al. (2015) to ensure that every pot has a successfully growing plant.
Acknowledgments
The authors thank Dr. L. Schreiber (University of Bonn, Germany) for suggesting the construction of the surface abrader. The methods used in this publication were first described in Schönherr and Lendzian (1981) and first adapted to petals by Buschhaus et al. (2015). Generous support to R. Jetter has been provided by the Natural Sciences and Engineering Research Council (Canada), the Canada Research Chairs Program, and the Canada Foundation for Innovation.
References
- Baur, P. (2008). Lognormal distribution of water permeability and organic solute mobility in plant cuticles. Plant Cell Environ 20(2): 167-177.
- Buschhaus, C., Hager, D. and Jetter, R. (2015). Wax layers on Cosmos bipinnatus petals contribute unequally to total petal water resistance. Plant Physiol 167(1): 80-88.
- Schreiber, L. and Schönherr, J. (2009). Water and solute permeability of plant cuticles. Springer-Verlag Berlin Heidelberg.
- Schönherr, J. and Lendzian, K. (1981). A simple and inexpensive method of measuring water permeability of isolated plant cuticular membranes. Z Pflanzenphysiol 102(4): 321-327.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Jetter, R. and Buschhaus, C. (2015). Quantifying the Permeability of the Apoplastic Water Barrier in Cosmos Petals. Bio-protocol 5(22): e1652. DOI: 10.21769/BioProtoc.1652.
- Buschhaus, C., Hager, D. and Jetter, R. (2015). Wax layers on Cosmos bipinnatus petals contribute unequally to total petal water resistance. Plant Physiol 167(1): 80-88.
Category
Plant Science > Plant physiology > Abiotic stress
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









