- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Culture of Megakaryocytes from Human Peripheral Blood Mononuclear Cells
Published: Vol 5, Iss 21, Nov 5, 2015 DOI: 10.21769/BioProtoc.1639 Views: 26739
Reviewed by: Ralph BottcherSalma HasanAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Selective Enrichment and Identification of Cerebrospinal Fluid-Contacting Neurons In Vitro via PKD2L1 Promoter-Driven Lentiviral System
Wei Tan [...] Qing Li
Nov 20, 2025 1343 Views

Revisiting Primary Microglia Isolation Protocol: An Improved Method for Microglia Extraction
Jianwei Li [...] Guohui Lu
Dec 5, 2025 1475 Views

Optimization of Adipogenic Differentiation Protocol for Murine and Human Cell Culture Models
Junwan Fan [...] Wenyan He
Jan 20, 2026 231 Views
Abstract
Megakaryocytes are the precursor cells of platelets and are bona fide resident cells in the bone marrow but extremely low in numbers (~1% of total nucleated cells). Upon terminal differentiation, megakaryocytes increase their size, become polyploid and develop a demarcation membrane system. Mature megakaryocytes form proplatelets, which are cytoplasmic extensions that protrude through the endothelial cell layer of venous sinusoids within the bone marrow, entering into the blood circulation and, subsequently, releasing platelets. Despite limited in numbers, megakaryocytes have been successfully isolated from bone marrow (Tolhurst et al., 2012), adult peripheral blood (Mazur et al., 1990; Thornton et al., 1999), cord blood (Sun et al., 2004) and also from embryonic stem cells (Pick et al., 2013; Eto et al., 2002). These procedures rely on immunostaining using antibodies against megakaryocyte surface markers (i.e. CD41 or CD42b) to isolate an enriched population of megakaryocytes. Here, we describe a culture method wherein megakaryocytes can be grown and differentiated in vitro from human peripheral blood mononuclear cells (PBMCs) directly without the need of initial isolation of CD34+ cells. This method is based on a previously published culture method of human erythroid progenitor cells from PBMCs (Borg et al., 2010; Leberbauer et al., 2005). Although the purity of megakaryocytes is not 100% in this culture method, an enriched fraction of megakaryocytes can be further isolated using BSA gradient or cell-sorting techniques. In addition, our method offers the possibility to freeze the cultures after minimal expansion of yet undifferentiated megakaryocytes, which will yield equal megakaryocyte cultures after thawing when compared to fresh uninterrupted cultures. As this has been proven difficult with CD34+ sorted pluripotent cells, it allows managing samples and to perform downstream analysis when human material is not always available.
Materials and Reagents
Note: *These materials/reagents can be replaced by similar alternatives.
- NUNC tubes-50 ml (Thermo Fisher Scientific, catalog number: 339651 )*
- Whole blood (aseptic) in anticoagulant (~ 50 ml volume)
- Phosphate buffer saline (PBS) (pH 7.4)
- Trisodium Citrate Dihydrate (TNC) buffer (38 gram/L demi water, pH 7.0) (Sigma-Aldrich, catalog number: S1804 )
- Percoll® (GE Healthcare, catalog number: 17-0891-09 )
- StemSpanTM SFEM (500 ml) (STEMCELL technologies, catalog number: 09650 )
- Lipids Cholesterol Rich from adult bovine serum (Sigma-Aldrich, catalog number: L4646 )*
- Penicillin-Streptomycin (Sigma-Aldrich, catalog number: P4333 )*
- Recombinant human stem cell factor (SCF) (Life technologies, catalog number: PHC2115 )*
Note: Currently, it is “Thermo Fisher Scientific, GibcoTM, catalog number: PHC2115”. - Recombinant human thrombopoietin (TPO) (Life technologies, catalog number: PHC9514 )*
Note: Currently, it is “Thermo Fisher Scientific, GibcoTM, catalog number: PHC9514”. - Recombinant human Erythropoietin (EPO) (R&D Systems, catalog number: 287-TC-500 )*
- (Optional) Recombinant Human Flt3-Ligand (FLT3-L) (Peprotech, catalog number: 30019 )*
- Culture media (see Recipes)
- Basis medium (see Recipes)
- Phase I medium (expansion) (see Recipes)
- Phase II medium (commitment) (see Recipes)
- Phase III medium (differentiation) (see Recipes)
- Basis medium (see Recipes)
Equipment
Note: *Indicated equipment can be replaced by similar alternatives.
- Centrifuge-Rotanta 460 (Hettich, catalog number: 5650 )*
- CASY Cell counter (Roche, model: CASY TTC )*
Procedure
- Start with approximately 50 ml of whole blood, recently collected using a closed system (sterile) from a healthy donor or a patient by a trained phlebotomist under consent. It is advisable to start the culture on the day of collection, but 1-2 days after extraction is also possible (although with donor variation). We normally use citrate phosphate dextrose, but EDTA and heparin are also compatible with this method.
- Centrifuge blood at 300 x g (1,000 rpm) for 15 min (using brake 3) at room temperature.
- Discard the Platelet Rich Plasma (PRP). Collect the buffy ring and also a little bit of red cell layer by pipetting, and mix 1:1 with PBS/TNC buffer in 50 ml tubes (Figure 1).
- Pipette carefully the diluted buffy ring in a proportion of 25 ml on top of 15 ml of Percoll® (1.076 g/ml) in 50 ml Nunc tubes. Centrifuge for 20 min at 1,220 x g or 2,400 rpm (accelerator 3, brake 3, room temperature).
- Discard upper layer (plasma) and take the buffy ring (where the peripheral blood mononuclear cells -PBMCs- are) that lays above the Percoll layer, avoiding the red cell lower layer (Figure 1).
- Wash PBMCs by adding 1:1 volume of PBS and centrifuge at 475 x g or 1, 500 rpm for 5 min, resuspend in the same volume of PBS.

Figure 1. Isolation of peripheral blood mononuclear cells (PBMCs). Blood is centrifuged and the buffy ring is diluted 1:1 with PBS/TNC, and added to a proportional volume of Percoll as described. After centrifugation, distinct layers are separated based on density, including plasma (yellow), buffy layer containing PBMCs (purple), Percoll (beige), and RBCs and granulocytes (red). Buffy layer was carefully isolated for further culture of megakaryocytes. - Count the cells using a cell counter. Resuspend to a density of 1-2 x 106 cells/ml in Phase I culture medium (preferably, grow cells initially in a standard 12-well culture plate).
- Observe cells every day. A healthy culture is characterized by round, healthy 7-10 μm cells, which grow single in suspension or in clumps (see Figure 2B). Medium should be refreshed every 2-3 days (for this, cells are collected, centrifuged at 475 x g or 1,500 rpm for 5 min at room temperature and resuspended in fresh medium). Dead cells and cell debri are washed out or diluted once the culture is refreshed with new medium, which helps to the welfare of the culture.
- Wash/refresh the culture at day 3-4 if necessary, and re-culture in fresh medium, or dilute in ½ volume of Phase I medium, depending on cell numbers, which should not exceed 4 x 106 cells/ml.
- From day 6-8, cells can be cultured in Phase II culture medium. In general, the duration of the culture in Phase II should not be longer than 4 days.
- Observe cells every day. Wash the culture after 3 days if necessary, and re-culture in fresh medium, or dilute in ½ volume of Phase II medium, depending on cell numbers.
- From day 9-12, cells can be cultured in Phase III culture medium. The start of Phase III depends on the culture development during Phase II, i.e. welfare of the culture and the majority of cells reaching a cell diameter > 13 μm (see Figures 2B and 3B).
- There will be appearance of bigger cells in the culture at day 13-14 or earlier and there should be gradually increasing numbers of these cells. Observe the culture every day, because depending on donor, some megakaryocytes will undergo terminal differentiation and start forming proplatelets, which implies losing cells due to cell death as well. Morphology and growth is subject to donor variability. It is estimated that from a starting culture with 50 x 106 cells, there will be around 50 x 106 megakaryocytes in average, with donor variation. This means that the culture first expands 2-4 times, and then, only 25-50% of the cells mature into megakaryocytes. See Figure 2 for an overview of the dynamics of the culture in terms of morphology and surface marker expression as measured by flow cytometer.


Figure 2. PBMC-derived megakaryocyte culture. A. Flow cytometry analysis. A cocktail of antibodies against megakaryocyte surface markers is used to distinguish differentiation stages in human megakaryocyte cultures. The expression of these markers through maturation is depicted below. B. Cell morphology. Cells were observed under the bright field microscope and pictures were taken at day 8, day 12 and day 16 of culture.
Important notes:- Although we present this culture method with the advantage to culture and differentiate megakaryocytes from PBMCs without the need of CD34+ cell selection, it goes without saying that it can be also applied to sorted CD34+ cells (derived from cord blood, bone marrow or peripheral blood).
- It is possible to freeze the cultures during the expansion phase: If needed, cells can be frozen between day 3 and day 6. Cells can be frozen in 10% DMSO in fetal bovine or calf serum (FBS or FCS) and placed at -80 °C in a freezing container overnight to allow gradual temperature drop before transferring them to liquid nitrogen for long-term storage. Upon thawing, culture cells for a couple of days in Phase I and then continue as normal towards Phase II and III. The culture will resume very quickly at this stage at which it was frozen and the growth will be very similar to the original culture in terms of cell numbers, morphology and expression of MK-specific surface markers as analyzed by flow cytometry (Figure 3).
- Although we present this culture method with the advantage to culture and differentiate megakaryocytes from PBMCs without the need of CD34+ cell selection, it goes without saying that it can be also applied to sorted CD34+ cells (derived from cord blood, bone marrow or peripheral blood).
Optional complementary techniques
Materials and Reagents
- Bovine serum albumin-100G (BSA) (Sigma-Aldrich, catalog number: A9647)*
- Conjugated antibodies for flow cytometry analysis:
- CD41a PerCP cy5.5 (CD41) (BD Biosciences, catalog number: 333148)*
- CD31 Pacific Blue (CD31) (Biolegend, catalog number: 303114)*
- cKIT PE-Cy7 (KIT) (Biolegend, catalog number: 313212)*
- CD42b APC (CD42b) (BD Biosciences, catalog number: 551061)*
- CD71 FITC (CD71) (BD Biosciences, catalog number: 555536)*
- CD61 FITC (CD61) (Sanquin, catalog number: M1592)*
- CD49b PE (CD49b) (BD Biosciences, catalog number: 555669)*
- CD41a PerCP cy5.5 (CD41) (BD Biosciences, catalog number: 333148)*
Equipment
- EasySepTM Magnet (STEMCELL Technologies)
- Flow Cytometer (LSRII + HTS) (BD Biosciences)*
- Fluorescent Microscope (Life Technologies, model: EVOS FL imaging system)*
Note: Currently, it is “Thermo Fisher Scientific, model: EVOS FL imaging system”.
Note: *These reagents/equipment can be replaced by similar alternatives.
Procedure
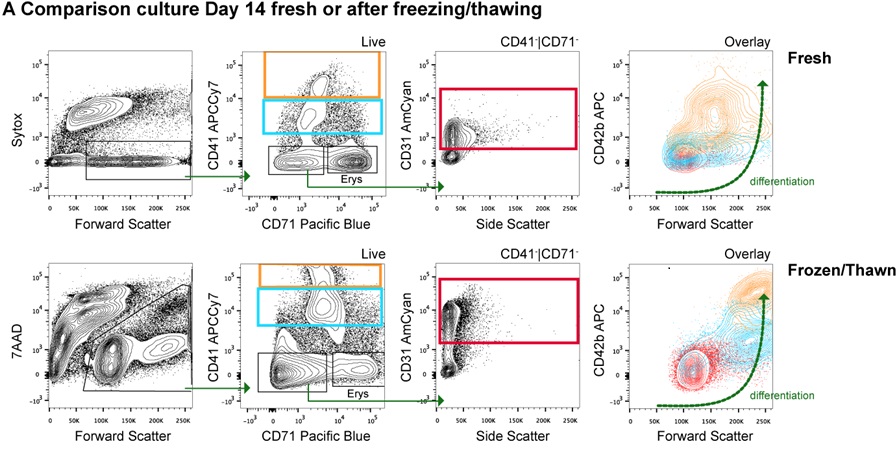

Figure 3. PBMC-derived megakaryocyte culture variations. A. Fresh vs. Phase I-frozen PBMC-derived megakaryocyte cultures: Flow cytometry analysis. A cocktail of antibodies against megakaryocyte surface markers is used to distinguish differentiation stages in human megakaryocyte cultures. The profiles from both conditions (Fresh vs. Phase I-Frozen) look similar and suggest that megakaryocytes can be successfully grown with this culture method, which allows freezing of cultures during the first days of culture Phase I. B. Cell morphology of cultures derived fromCD34+ sorted cells instead of PBMCs. Cells were observed under the bright field microscope and pictures were taken. The morphology of megakaryocytes from cultured CD34+ sorted cells is similar to that from cultured total PBMCs.
- BSA gradient
- If you need to collect a purer fraction of megakaryocytes (since the cultures are always heterogeneous), you can do a BSA gradient to collect the bigger cells by sedimentation and proceed with the enriched fraction for further analysis.
- A BSA gradient (0%, 1.5% and 3%) can be used to further isolate relatively bigger size megakaryocytes as depicted in Figure 4. If the amount of cells is above 10 million, perform the BSA gradient on a 50 ml tube with 5 ml volume for each layer. If the amount of cells is lower than10 million, perform the BSA gradient on a 15 ml tube with 3 ml volume for each layer.
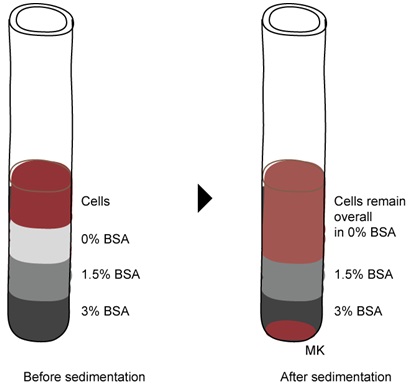
Figure 4. Enrichment of cultured megakaryocytes using a BSA gradient. Cells from the Phase III medium were allowed to sediment through a BSA gradient for 15-30 min. Megakaryocytes will pellet at the bottom and can be collected in PBS. - Allow cells to sediment for 15-30 min at 0 x g (no centrifugation) so that megakaryocytes will pellet at the bottom of the tube.
Collect megakaryocytes with PBS, wash once again at low speed (100 x g or 700 rpm) and proceed further with the downstream analysis. - Alternatively, megakaryocytes can be retrieved by magnetic separation (EasySepTM Magnet) based on positive selection using an antibody (e.g. CD41-FITC) or by FACS sorting depending on the downstream experimental procedure.
- If you need to collect a purer fraction of megakaryocytes (since the cultures are always heterogeneous), you can do a BSA gradient to collect the bigger cells by sedimentation and proceed with the enriched fraction for further analysis.
- Flow cytometry based megakaryocyte differentiation
Basic flow cytometry analysis can be performed using a cocktail of antibodies that allow the analysis of the differentiation stages of megakaryocytes in culture. Figures 2-3 show examples of the gating strategies, and the sequence of expression of surface markers during megakaryocyte differentiation.
Recipes
- Culture media
- Basis medium
StemSpan (500 ml)
Cholesterol rich lipid mix (Add 2 ml in 500 ml StemSpan medium)
1% Pen/Strep - Phase I medium (expansion)
Basis medium supplemented with:
SCF 100 ng/ml
TPO 30 ng/ml
EPO 0.5 U/ml
FLT3-L 50 ng/ml (optional, although it favors expansion) - Phase II medium (commitment)
Basis medium supplemented with:
SCF 50 ng/ml
TPO 50 ng/ml
EPO 0.5 U/ml
FLT3-L 25 ng/ml (optional, although it favors expansion) - Phase III medium (differentiation)
Basis medium supplemented with:
TPO 100 ng/ml
- Basis medium
Acknowledgments
This protocol was adapted from previous culture method (Borg et al., 2010; Leberbauer et al., 2005) on human erythroid progenitor cells and was developed by V.S., P.P., and L.G. Blood samples were obtained under informed consent, after approval by our institute medical ethics committee in accordance with the 1964 Declaration of Helsinki. This work was partly supported by an I+D Excelencia 2014 grant (SAF2014-55231-P; Ministerio de Economía y Competitividad -Spain- y Fondos Feder, L.G. and P.P.) and a Ramón y Cajal Fellowship (RYC-2013-12587; Ministerio de Economía y Competitividad -Spain-, L.G.).
References
- Borg, J., Papadopoulos, P., Georgitsi, M., Gutierrez, L., Grech, G., Fanis, P., Phylactides, M., Verkerk, A. J., van der Spek, P. J., Scerri, C. A., Cassar, W., Galdies, R., van Ijcken, W., Ozgur, Z., Gillemans, N., Hou, J., Bugeja, M., Grosveld, F. G., von Lindern, M., Felice, A. E., Patrinos, G. P. and Philipsen, S. (2010). Haploinsufficiency for the erythroid transcription factor KLF1 causes hereditary persistence of fetal hemoglobin. Nat Genet 42(9): 801-805.
- Eto, K., Murphy, R., Kerrigan, S. W., Bertoni, A., Stuhlmann, H., Nakano, T., Leavitt, A. D. and Shattil, S. J. (2002). Megakaryocytes derived from embryonic stem cells implicate CalDAG-GEFI in integrin signaling. Proc Natl Acad Sci U S A 99(20): 12819-12824.
- Leberbauer, C., Boulme, F., Unfried, G., Huber, J., Beug, H. and Mullner, E. W. (2005). Different steroids co-regulate long-term expansion versus terminal differentiation in primary human erythroid progenitors. Blood 105(1): 85-94.
- Mazur, E. M., Basilico, D., Newton, J. L., Cohen, J. L., Charland, C., Sohl, P. A. and Narendran, A. (1990). Isolation of large numbers of enriched human megakaryocytes from liquid cultures of normal peripheral blood progenitor cells. Blood 76(9): 1771-1782.
- Pick, M., Azzola, L., Osborne, E., Stanley, E. G. and Elefanty, A. G. (2013). Generation of megakaryocytic progenitors from human embryonic stem cells in a feeder- and serum-free medium. PLoS One 8(2): e55530.
- Sun, L., Tan, P., Yap, C., Hwang, W., Koh, L. P., Lim, C. K. and Aw, S. E. (2004). In vitro biological characteristics of human cord blood-derived megakaryocytes. Ann Acad Med Singapore 33(5): 570-575.
- Thornton, M. A. and Poncz, M. (1999). In vitro expansion of megakaryocytes from peripheral blood hematopoietic progenitors. Methods Mol Med 31: 337-345.
- Tolhurst, G., Carter, R. N., Miller, N. and Mahaut-Smith, M. P. (2012). Purification of native bone marrow megakaryocytes for studies of gene expression. Methods Mol Biol 788: 259-273.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Salunkhe, V., Papadopoulos, P. and Gutiérrez, L. (2015). Culture of Megakaryocytes from Human Peripheral Blood Mononuclear Cells. Bio-protocol 5(21): e1639. DOI: 10.21769/BioProtoc.1639.
Category
Cell Biology > Cell isolation and culture > Cell differentiation
Cell Biology > Cell isolation and culture > Cell isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link