- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Kinetic Analysis of Monoclonal Antibody Binding to HIV-1 gp120-derived Hyperglycosylated Cores
Published: Vol 5, Iss 20, Oct 5, 2015 DOI: 10.21769/BioProtoc.1615 Views: 8539
Reviewed by: Savita NairAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Surface Plasmon Resonance Method to Study HCV NS5B Inhibitors
Melanie Wong and Giuseppe A. Papalia
Feb 20, 2014 9493 Views

Large-scale Purification of Type III Toxin-antitoxin Ribonucleoprotein Complex and its Components from Escherichia coli for Biophysical Studies
Parthasarathy Manikandan [...] Mahavir Singh
Jul 5, 2023 2174 Views

Flotation Assay With Fluorescence Readout to Study Membrane Association of the Enteroviral Peripheral Membrane Protein 2C
Kasturika Shankar [...] Lars-Anders Carlson
Apr 5, 2025 1621 Views
Abstract
Kinetic analysis of antibodies is one of the important studies for characterization of antibodies and screening of ligands. In our recent study (Ingale et al., 2014), we compared the antigenic profiles and binding characteristics of four HIV-1 envelope glycoprotein (Env) core immunogens using multiple monoclonal antibodies by Bio-Layer Light Interferometry (BLI). This technology enables real-time analysis of interactions on the surface of a fiber optic biosensor by accurately measuring kinetic constants such as Ka, Kd, and KD in a 96-well format.
Materials and Reagents
- 96-well black bottom plates (Greiner Bio-One GmbH, catalog number: 655209 )
- Anti-human Fc capture biosensors (Pall Corporation, ForteBio®, catalog number: 18-5060 )
- Phosphate buffered saline (Life Technologies, GibcoTM, catalog number: 100-10023 )
- Monoclonal antibodies and hyperglycosylated core analytes (expressed in-house)
Equipment
- Octet Red system (Pall Corporation, ForteBio®, part number: 30-5048 )
Software
- Data Analysis 6.2 evaluation software (Forte Bio)
Procedure
Biosensors are tips coated with proprietary biocompatible matrix that can bind to Fc region of human immunoglobulin with minimal non-specific binding. In a separate black bottom plate, the biosensors were hydrated in 200 μl PBS for 10 min at room temperature prior to the experiment. The sample plate was generated according to the template shown in Figure 1. The buffer wells were filled with 200 μl PBS. Selected monoclonal antibodies were diluted to 5 μg/ml in PBS and dispensed at a volume of 200 μl into each well of the loading column as indicated in the template. The H1 well was filled with 200 μl PBS. The analyte (HIV envelope core glycoprotein is used in the example shown in Figure 1) was 2-fold serially diluted in PBS starting from 1 μM to 31.25 nM in a separate plate and transferred in a 200 μl volume in the “association column” of the template as shown (Figure 1). The sensor plate (plate with bio-sensors) and sample plate (plate with other reagents, as shown in Figure 1) were then inserted into the Octet Red machine and a basic kinetic measurement experiment was performed as follows:
- The sensors were dipped in buffer for equilibration.
- The sensors were transferred to the sensor plate and monoclonal antibodies were captured on the sensors at 1,000 rpm for 60 sec.
- Sensors were washed in PBS and a stable baseline was achieved at 1,000 rpm for 60 sec.
- The antibody-immobilized sensors were immersed in analyte-containing wells for 600 sec at 1,000 rpm to allow potential association of analyte with a given monoclonal antibody.
- Bio-sensors were next transferred to and immersed in wells containing a volume of 200 μl PBS at 1,000 rpm for 600 sec for the dissociation of the bound analyte.
During each run two reference sensor were included, first where at the association step the sensors were immersed in PBS buffer as an analyte control (to be used for subtraction during analysis of the raw data) and second where no antibody was immobilized on the sensor and a 500 nM analyte was used during the association phase to assess the level of non-specific binding of analyte to the anti-human Fc capture sensors, if any such interactions were detected.
The data was analyzed with Data Analysis 6.2 evaluation software. The data was processed using analyte control reference well for subtraction from the experimental data curves, aligning Y-axis to the baseline, and applying Savitzky-Golay as the filtering process. Next, the association and dissociation response curves were plotted using 1:1 model and global full curve fitting to compute the Ka, Kd, and KD values.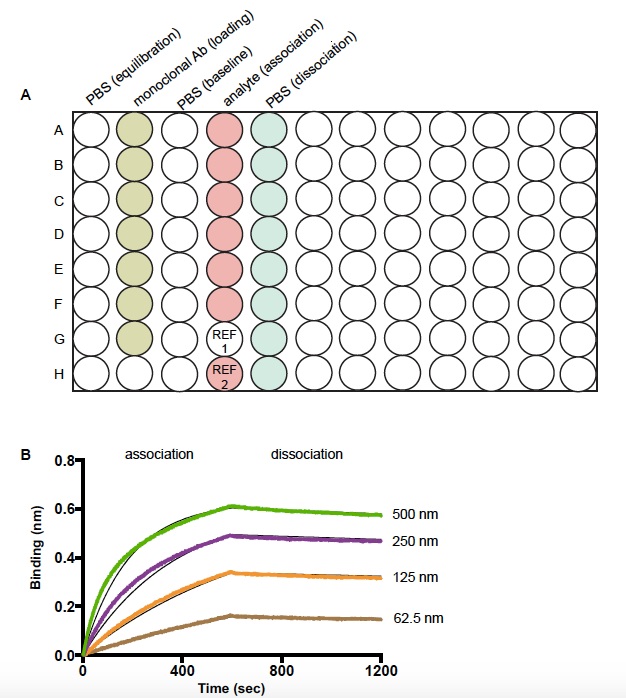
Figure 1. Schematic diagram of sample plate and representative data of a kinetic experiment by bio-layer interferometry (BLI). A. Sample plate setup for measuring the kinetics of binding interaction between a monoclonal antibody and its ligand. REF 1 well is filled with PBS and used for reference subtraction. REF 2 well is a control experiment to check the non-specific binding of an analyte to the Fc capture sensors. B. The binding kinetics of a broadly neutralizing HIV 1 monoclonal antibody to a stable core envelope protein at various concentrations of the analyte. The black lines represent the theoretical Langmuir fits generated by 1:1 binding kinetics. The colored lines are the experimental curves for association and dissociation of the binding event.
Acknowledgments
This protocol was previously used in Ingale et al. (2014).
References
- Ingale, J., Tran, K., Kong, L., Dey, B., McKee, K., Schief, W., Kwong, P. D., Mascola, J. R. and Wyatt, R. T. (2014). Hyperglycosylated stable core immunogens designed to present the CD4 binding site are preferentially recognized by broadly neutralizing antibodies. J Virol 88(24): 14002-14016.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Ingale, J. and Wyatt, R. T. (2015). Kinetic Analysis of Monoclonal Antibody Binding to HIV-1 gp120-derived Hyperglycosylated Cores. Bio-protocol 5(20): e1615. DOI: 10.21769/BioProtoc.1615.
Category
Immunology > Antibody analysis > Antibody-antigen interaction
Microbiology > Microbial biochemistry > Protein > Interaction
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









