- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Quantification of Callose Deposition in Plant Leaves
Published: Vol 5, Iss 19, Oct 5, 2015 DOI: 10.21769/BioProtoc.1610 Views: 16679
Reviewed by: Tie LiuFanglian HeAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Quick Method to Quantify Iron in Arabidopsis Seedlings
Chandan Kumar Gautam [...] Wolfgang Schmidt
Mar 5, 2022 3932 Views

Isolation of Intact Vacuoles from Arabidopsis Root Protoplasts and Elemental Analysis
Chuanfeng Ju [...] Zhenqian Zhang
Mar 5, 2023 2053 Views

High-Performance Liquid Chromatography Quantification of Glyphosate, Aminomethylphosphonic Acid, and Ascorbate in Culture Medium and Microalgal Cells
Juan Manuel Ostera [...] Gabriela Malanga
Apr 5, 2025 1182 Views
Abstract
Callose is an amorphous homopolymer, composed of β-1, 3-glucan, which is widespread in higher plants. Callose is involved in multiple aspects of plant growth and development. It is synthetized in plants at the cell plate during cytokinesis, in several stages during pollen development and is deposited at plasmodesmata to regulate the cell-to-cell movement of molecules. Moreover, it is produced in response to multiple biotic and abiotic stresses (Chen and Kim, 2009). Callose is considered to act as a physical barrier by strengthening the plant cell well to slow pathogen infection and to contribute to the plant’s innate immunity. Thus the callose staining method is useful to quantify activity of plant immunity. In addition, this staining can be used to visualize structures in plant tissue, where the callose may be implied whether during the development of plants or response against pathogen infection. This method is based on the use of methyl blue which reacts with (1→3)-β-glucans to give a brilliant yellow fluorescence in UV light. Moreover, calcofluor stains chitin present in fungal cell membranes and also binds to cellulose at locations where the cuticle is damaged.
Keywords: CalloseMaterials and Reagents
- Plant materials: Arabidopsis thaliana cotyledons and leaves, Solanum lycopersicum leaves or Citrus leaves
- 96% ethanol
- Na2HPO4.2H2O
- NaH2PO4.2H2O
- Methyl blue (Sigma-Aldrich, catalog number: M6900 )
- Sodium phosphate buffer (see Recipes)
- Methyl blue solution 0.5% and 0.05% (see Recipes)
Optional
- Trizma® base (Sigma-Aldrich, catalog number: T1503 )
- Fluorescent Brightener 28 (synonym Calcofluor white) (Sigma-Aldrich, catalog number: F3543 )
- HCl
- Tris.HCl buffer (see Recipes)
- Fluorescent brightener solution 0.01% (see Recipes)
Equipment
- Leica IRB epifluorescence microscope with UV filter (BP 340 to 380 nm, LP 425 nm) equipped with a Leica DC300F camera (Leica Microsystems, model: Leica IRB )
Software
- Digital photographs analysis software like GIMP (http://www.gimp.org) or Adobe Photoshop (adobe.com) are recommended
Procedure
Note: Staining with fluorescent Brightener is optional. This will stain the chitin present in the sample, and can be useful to stain the fungus. However, the use of this staining can mask the fluorescence emitted by callose. For this reason, if the goal of the experiment is to quantify callose deposition, the use of fluorescent Brightener is not recommended.
- Staining procedure
- This procedure can be used for staining Arabidopsis thaliana cotyledons and leaves, Solanum lycopersicum leaves or Citrus leaves with little variation according to tissue toughness.
- Sample the entire leaves or the cotyledons from the plant and place them in 50 ml tubes containing 96% ethanol for chlorophyll removal. Distain the samples in 96% ethanol until they appear completely white (time can vary between 1 day for small leaves like the ones of Arabidopsis till one week for citrus leaves). The saturated distaining 96% ethanol can be replaced, if necessary.
- Rehydrate the samples in sodium phosphate buffer for 30 min.
- Discard the phosphate buffer and cover the sample with 0.05% Methyl blue solution.
Optional: Add 1/5 of fluorescent brightener solution (1 ml per each 4 ml of methyl blue solution used in the previous step). - Incubate for 30 min and discard the solution.
- For Arabidopsis cotyledons go to step A8. Callose can be observed after 3-4 h of incubation with 0.05% Methyl blue as previously described by Luna et al. (2011).
- Cover the sample with 0.5% Methyl blue solution and incubate for 24 h in darkness. (Time may vary depending on the sample. Thought samples, such as citrus leaves, may require a vacuum stroke of 2 min and up to 7 days of staining.)
- For microscope observation, mount the samples on slides with the adaxial surface up using freshly prepared 0.05% Methyl blue.
- Observe the samples with an epifluorescence microscope with UV filter. Stained callose appears as fluorescent yellow spots (Figure 1).
Note: Be aware to use all the time the same augmentation.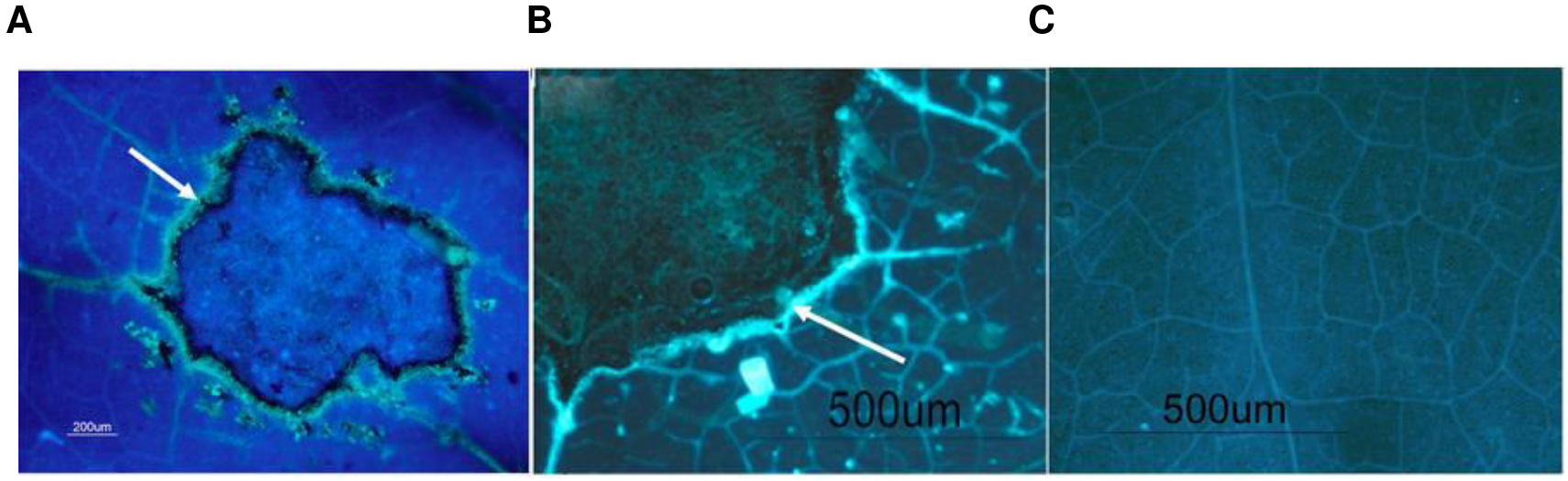
Figure 1. Representative picture of callose deposition in tomato leaves upon Botrytis cinerea infection with fluorescent brightener solution (A) and without fluorescent brightener solution (B). The arrow highlights callose. Representative picture of control leaves (C).
- This procedure can be used for staining Arabidopsis thaliana cotyledons and leaves, Solanum lycopersicum leaves or Citrus leaves with little variation according to tissue toughness.
- Image processing
- Open the images with the photography software (Figure 2).
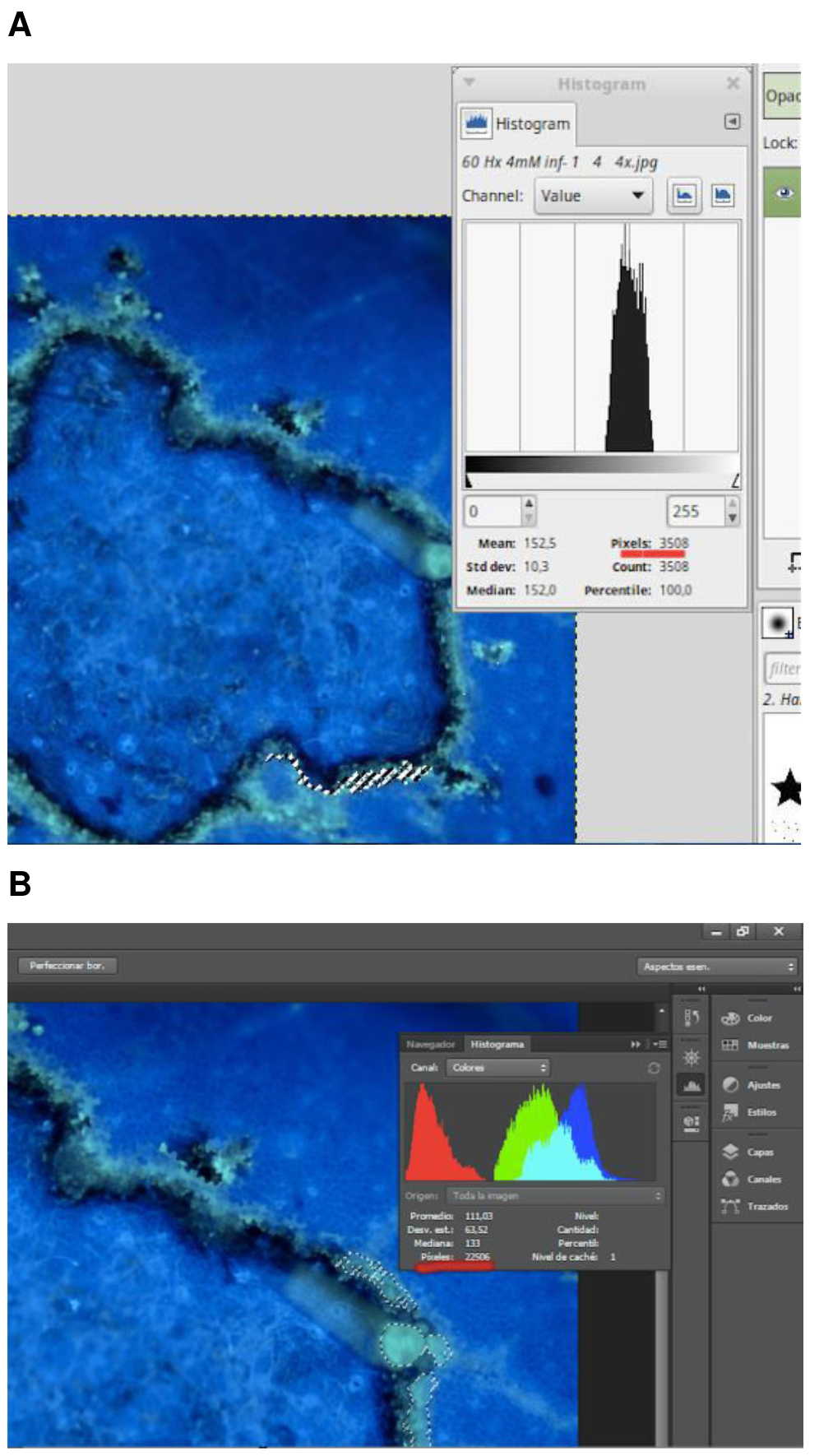
Figure 2. Yellow spots corresponding to stained callose analyzed for the number of pixels using A. GIMP (GNU Image Manipulation Program) or B. Adobe Photoshop - Select the brilliant yellow pixels that correspond to callose using a Magic wand tool or select by color range.
- Use the histogram window to determine the number of pixels.
- Represent callose intensity as average number of bright pixels/number of total pixels.
In the case of cotyledons it is possible to calculate the yellow pixels per leaf area.
See Video for more details.Video 1. Quantification process using GimpVideo 2. Quantification process with photoshop
- Open the images with the photography software (Figure 2).
Recipes
- Sodium phosphate buffer (0.07 M, pH=9)
Dissolve 12.46 g of Na2HPO4.2H2O in 1 L of distilled water
Dissolve 0.966 g of NaH2PO4.2H2O in 100 ml of distilled water
Adjust the pH of Na2HPO4.2H2O solution to 9, using the solution of NaH2PO4.2H2O - Methyl blue solution freshly prepared
For 0.5% solution: Dissolve 0.5 g of Methyl Blue in 100 ml of sodium phosphate buffer
For 0.05% solution: Dissolve 0.05 g of Methyl Blue in 100 ml of sodium phosphate buffer
Optional solutions
- Tris.HCl buffer (0.1 M, pH =8.5)
Dissolve 0.6057 g of Tris base in 50 ml of distilled water and adjust the pH at 8.5 with HCl - Fluorescent brightener solution
Dissolve 0.0125 g of Fluorescent Brightener 28 in 50 ml of Tris buffer
Stored the solution in dark at room temperature
Acknowledgments
Authors thank Universitat Jaume I and the National R&D Plan (AGL2010-22300-C03-02, Spain for funding support.
References
- Chen, X. Y. and Kim, J. Y. (2009). Callose synthesis in higher plants. Plant Signal Behav 4(6): 489-492.
- Luna, E., Pastor, V., Robert, J., Flors, V., Mauch-Mani, B. and Ton, J. (2011). Callose deposition: a multifaceted plant defense response. Mol Plant Microbe Interact 24(2): 183-193.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Scalschi, L., Llorens, E., Camañes, G., Pastor, V., Fernández-Crespo, E., Flors, V., García-Agustín, P. and Vicedo, B. (2015). Quantification of Callose Deposition in Plant Leaves. Bio-protocol 5(19): e1610. DOI: 10.21769/BioProtoc.1610.
Category
Plant Science > Plant biochemistry > Other compound
Plant Science > Plant cell biology > Cell staining
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link












