- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
In vitro Studies: Inhibition of Nevirapine Metabolism by Nortriptyline in Hepatic Microsomes
Published: Vol 5, Iss 19, Oct 5, 2015 DOI: 10.21769/BioProtoc.1607 Views: 8745
Reviewed by: Vamseedhar RayaproluAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Analysis of the Effect of Sphingomyelinase on Rubella Virus Infectivity in Two Cell Lines
Noriyuki Otsuki [...] Makoto Takeda
Sep 5, 2018 6091 Views

Primer ID Next-Generation Sequencing for the Analysis of a Broad Spectrum Antiviral Induced Transition Mutations and Errors Rates in a Coronavirus Genome
Shuntai Zhou [...] Ronald Swanstrom
Mar 5, 2021 7470 Views

An Improved Focus-Forming Assay for Determination of the Dengue Virus Titer
Maharah Binte Abdul Mahid [...] Kitti Wing Ki Chan
Oct 20, 2024 2410 Views
Abstract
One of the most prevalent and interfering psychosocial comorbidities of HIV infection is clinical depression (22 to 45%). For this reason, a study of a possible interaction between the nonnucleoside reverse transcriptase inhibitor nevirapine (NVP) and the tricyclic antidepressant nortriptyline (NT) was carried out. In vitro studies with rat and human hepatic microsomes showed a marked inhibition of NVP metabolism by NT being more intense in rat than in human. The extrapolation of these results to humans suggests increased NVP side effects when both drugs are coadministered, but additional in vivo human studies are required to evaluate the clinical implication of this interaction.
This protocol describes a technique for detecting and measuring the inhibition of the nevirapine metabolism by nortriptyline in hepatic microsomes.
Materials and Reagents
- 10 ml polypropylene tube 16 x 95 mm (Deltalab, catalog number: 400900 )
- 5 ml ultracentrifuge tube 13 x 51 mm (Beckman Coulter, catalog number: 326819 )
- 1.5 ml screw cap vial 32 x 11.6 mm
- 7.5 ml screw cap tube 13 x 100 mm
- Wistar rat livers
- Saline solution (Laboratorios ERN, catalog number: 999790 )
- Protein Standard (2 mg BSA) (BD Biosciences, Falcon®, catalog number: P5619 )
Note: Currently, it is “Sigma-Aldrich, catalog number: P5619”. - Na2CO3 (EMD Millipore Corporation, catalog number: 106392 )
- Copper(II) sulfate pentahydrate (CuSO4.5H2O) (Sigma-Aldrich, catalog number: 209198 )
- Potassium sodium tartrate tetrahydrate (Sigma-Aldrich, catalog number: 217255 )
- Folin-Ciocalteu’s phenol reagent (EMD Millipore Corporation, catalog number: 109001 )
- Potassium phosphate monobasic (KH2PO4) (BD Biosciences, Falcon®, catalog number: 60229 )
Note: Currently, it is “Sigma-Aldrich, catalog number: 60229”. - Sodium phosphate dibasic (Na2HPO4) (Sigma-Aldrich, Falcon®, catalog number: 56814 )
- KCl (EMD Millipore Corporation, catalog number: 104936 )
- Ethylendiaminetetraacetic acid disodium salt-2-hydrate (EDTA) (Riedel-de Haën, catalog number: 34549 )
Note: Currently, it is “Sigma-Aldrich, FLUKA, catalog number: 34549 ”. - Sucrose (BD Biosciences, Falcon®, catalog number: 84100 )
Note: Currently, it is “Sigma-Aldrich, catalog number: 84100”. - K2HPO4.3H2O (AppliChem GmbH, catalog number: 122333 )
- Nevirapine (Viramune) (Boehringer Ingelheim)
- Methanol, HiPerSolv CHROMANORM for HPLC-Gradient Grade (VWR International, Prolabo, catalog number: 20864.320 )
- Dimethyl sulfoxide (DMSO) (minimum 99.5% GC, plant cell culture tested) (Sigma -Aldrich, catalog number: D4540 )
- Nortriptyline hydrochloride, minimum 98% TLC (Sigma-Aldrich, catalog number: N7261 )
- D-Glucose 6-phosphate disodium salt hydrate (Sigma-Aldrich, catalog number: G7250 )
- β-Nicotinamide adenine dinucleotide phosphate disodium salt (β-NADP) (Sigma-Aldrich, catalog number: 93205 )
- Glucose-6-phosphate dehydrogenase from Leuconostoc mesenteroides (Sigma-Aldrich, catalog number: G5760 )
- MgCl2.6H2O, BioReagent, suitable for cell culture, suitable for insect cell culture (Sigma-Aldrich, catalog number: M2393 )
- Acetonitrile (VWR, Prolabo, catalog number: 83639.320 )
- Human liver microsomes pooled from 50 different individual donors (Thermo Fisher Scientific, InvitrogenTM, catalog number: HMMCPL )
- Sodium hydroxide (extra pure, pellets) (Scharlab,S.L., catalog number: SO0420005P )
- Ethyl acetate (extra pure) (Scharlab,S.L., catalog number: AC01431000 )
- NaH2PO4.2H2O (Guinama, catalog number: 90790 )
- Triethylamine (Sigma-Aldrich, catalog number: T0886 )
- 50 mM sodium-potassium phosphate buffer (see Recipes)
- 0.1 M potassium phosphate buffer (see Recipes)
- Lowry reagent (see Recipes)
- 50 mM sodium phosphate monobasic buffer (see Recipes)
Equipment
- Surgical tools
- Scissors
- Spectrophotometer (Hitachi High-Technologies Corporation, model: U-2900 )
- 1 cm polystyrene spectrophotometer cuvette (Sigma-Aldrich, catalog number: C5219 )
- Thermostatic water bath
- Refrigerated centrifuge (Sigma Laborzentrifugen, model: 2K15 )
- Refrigerated ultracentrifuge (130,000 rpm) (Beckman Coulter, model: OptimaTM MAX ultracentrifuge )
- Tissue Grinder, Potter-Elvehjem type, 30 ml Glass Vessel and Plain Plunger (VWR, Prolabo, catalog number: 432-0204 and 89026-400 , respectively)
Procedure
- Inhibition of the nevirapine metabolism by nortriptyline in rat hepatic microsomes
- Isolate rat liver microsomes following these steps:
- Sacrifice a total of 5 Wistar rats (280-310 g) by isoflurane inhalation.
- Open the abdominal cavity of each rat, excise the liver and place it into a beaker containing saline solution.
- Wash the liver with saline solution and place it on a paper towel.
- Weigh the liver and cut it into small pieces at 4 °C.
- Place the liver into the glass vessel and add 5x its mass in volume of 50 mM sodium-potassium phosphate buffer (pH 7.4) containing 1.15% (wt/vol) KCl, 2 mM EDTA, and 0.25 M sucrose.
- Homogenize the liver with the tissue grinder until a complete homogeneous solution is obtained.
- Transfer the homogenate to 10 ml polypropylene tubes and centrifuge (4 °C) at 9,000 x g for 10 min.
- Transfer the supernatant to ultracentrifuge tubes and centrifuge (4 °C) at 150,000 x g for 1 h.
- Resuspend the microsomal pellet in 0.1 M potassium phosphate buffer (pH 7.4).
- Sacrifice a total of 5 Wistar rats (280-310 g) by isoflurane inhalation.
- Determine microsomal protein concentration following the method of Lowry et al. (1951):
- Prepare a series of dilutions of 2 mg bovine serum albumin in water, to give concentrations of 0.1 to 1 mg/ml.
- Add to 100 µl of each dilution of standard or sample containing microsomes and 2 ml of Lowry reagent to each 10 ml polypropylene tube.
- Incubate the tubes in the dark at room temperature for 15 min.
- Add 200 µl of an aqueous solution of Folin-Ciocalteu’s phenol reagent (1/2, vol/vol) to each tube.
- Incubate the tubes in the dark at room temperature for 30 min.
- Measure absorbance at 750 nm in 1 cm spectrophotometer cuvettes.
- Prepare a series of dilutions of 2 mg bovine serum albumin in water, to give concentrations of 0.1 to 1 mg/ml.
- Prepare a mixture in a 1.5 ml screw cap vial that contains the following compounds (per 100 µl):
- 10 µl of a solution of NVP in a mixture of water, methanol, and DMSO (final concentrations: 5 µg/ml NVP, 0.12% methanol, and 0.08% DMSO)
- 10 µl of NT solutions in water (final NT concentrations of 100, 200, 300, 500, 1,000, 2,000, 3,000, and 10,000 ng/ml) or 10 µl of H2O in the case of controls.
- 5 µl of a 20-mg/ml glucose-6-phosphate aqueous solution
- 5 µl of a 20-mg/ml β-NADP aqueous solution
- 10 µl of a glucose-6-phosphate dehydrogenase aqueous solution (10 IU/ml)
- 5 µl of a MgCl2 aqueous solution (13.4 mg/ml)
- Quantity sufficient to 100 µl of 0.1 M potassium phosphate buffer (pH 7.4)
- Rat hepatic microsomes previously isolated (equivalent to 0.1 mg of protein). With this addition the metabolic reaction is started
- 10 µl of a solution of NVP in a mixture of water, methanol, and DMSO (final concentrations: 5 µg/ml NVP, 0.12% methanol, and 0.08% DMSO)
- Incubate the test vials for 30 min at 37 °C in a water bath.
- Terminate the reaction in an ice bath by adding 100 µl of acetonitrile to the test vials.
- Centrifuge the vials at 2,000 x g for 5 min at 4 °C.
- Determine the amount of NVP in the supernatant liquid by high performance liquid chromatography (HPLC), using a C18 column and a UV detector set at 240 nm, as described previously (Usach et al., 2014).
- Isolate rat liver microsomes following these steps:
- Inhibition of nevirapine metabolite formation by nortriptyline in human hepatic microsomes
- Prepare a mixture in a 7.5 ml screw cap tube that contains the following compounds (per 100 µl):
- 20 µl of a solution of NVP in a mixture of water, methanol, and DMSO (final concentrations of 5 µg/ml NVP, 0.12% methanol, and 0.08% DMSO)
- 20 µl of NT solutions in water (final NT concentrations of 100, 1,000 and 10,000 ng/ml) or 10 µl of H2O in the case of controls.
- 10 µl of a 20-mg/ml glucose-6-phosphate aqueous solution
- 10 µl of a 20-mg/ml β-NADP aqueous solution
- 20 µl of a glucose-6-phosphate dehydrogenase aqueous solution (10 IU/ml)
- 10 µl of a MgCl2 aqueous solution (13.4 mg/ml)
- Quantity sufficient to 0.2 ml of 0.1 M potassium phosphate buffer (pH 7.4)
- Human hepatic microsomes (equivalent to 0.2 mg of protein). With this addition the metabolic reaction is started.
- 20 µl of a solution of NVP in a mixture of water, methanol, and DMSO (final concentrations of 5 µg/ml NVP, 0.12% methanol, and 0.08% DMSO)
- Incubate test tubes for 30 min at 37 °C in a water bath.
- Terminate the reaction in an ice bath by adding 25 µl of a 2 N sodium hydroxide solution and 5 ml of ethyl acetate to extract NVP and its metabolites.
- Shake the mixture and wait a few minutes until the two phases are completely separated.
- Transfer the supernatant to another 7.5 ml screw cap tube and evaporate it in a 40 °C water bath under a nitrogen stream.
- Repeat the extraction: Add 3 ml of ethyl acetate, shake the tube and evaporate the supernatant as before.
- Dissolve the dried residue into 100 µl of the mobile phase composed of acetonitrile and sodium phosphate monobasic buffer (50 mM [pH 4.6]) containing 0.1% (vol/vol) triethylamine (12/88, vol/vol).
- Centrifuge vials at 2,000 x g for 5 min at 4 °C.
- Determine the amount of NVP and its metabolites in the supernatant liquid by HPLC, using a C18 column and a UV detector set at 240 nm, as described previously (Usach, et al., 2014).
- Prepare a mixture in a 7.5 ml screw cap tube that contains the following compounds (per 100 µl):
Representative data
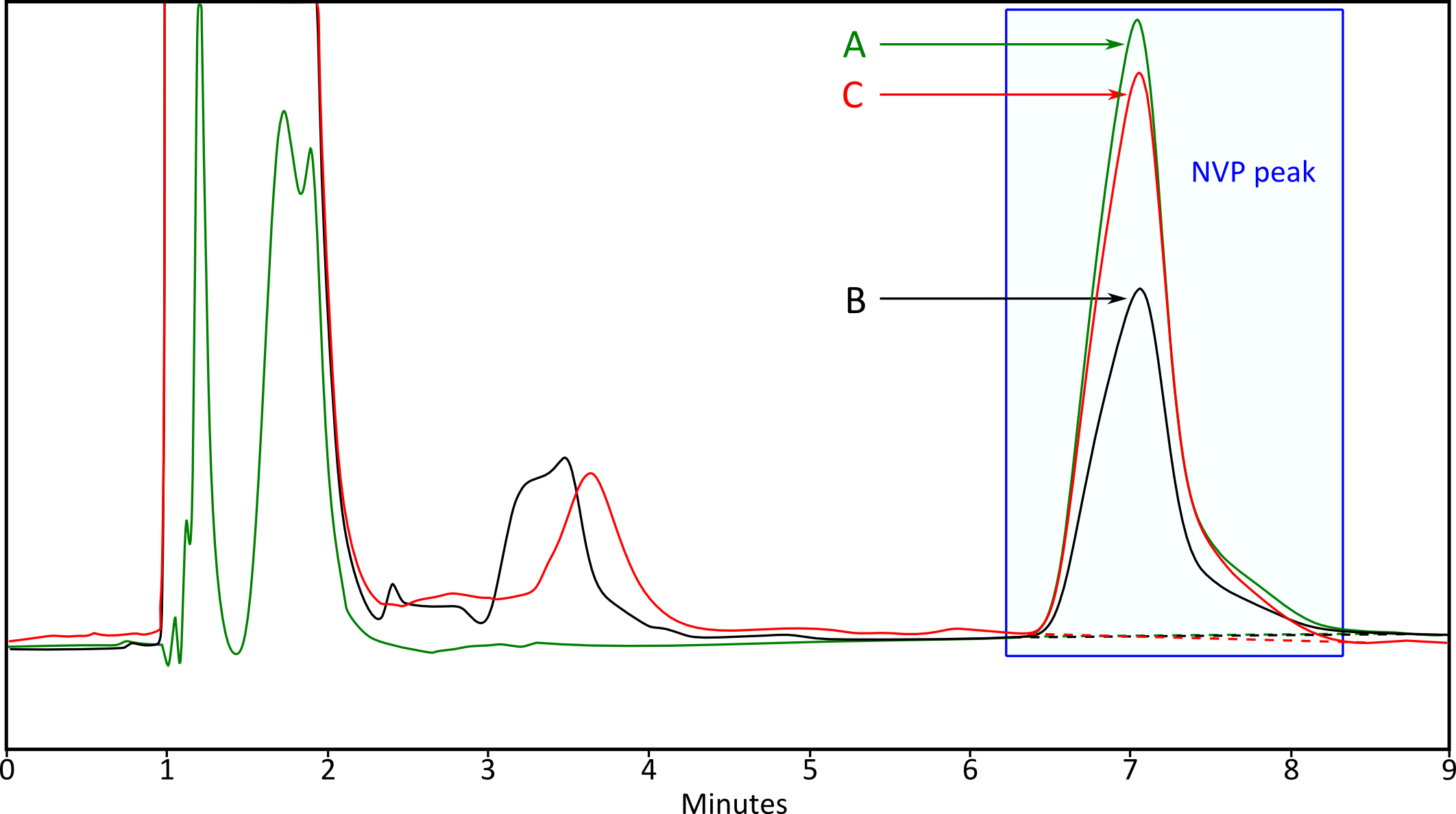
Figure 1. Representative HPLC-UV chromatogram of NVP metabolism in rat liver microsomes (NVP 5 µg/ml). A. Standard of NVP (no metabolism); B. NVP after 30 min at 37 °C; C. NVP and NT (10 µg/ml) after 30 min at 37 °C.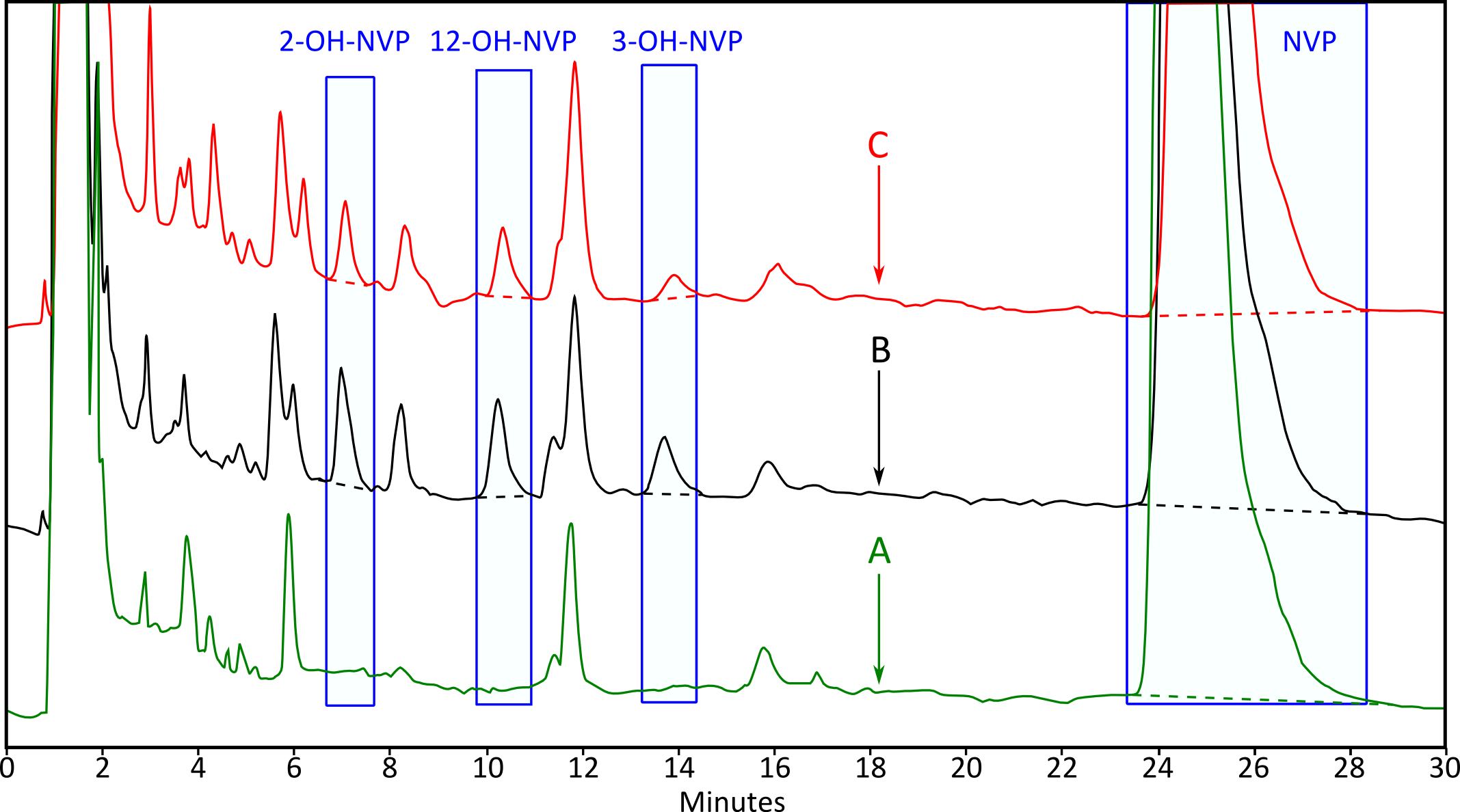
Figure 2. Representative HPLC-UV chromatogram of NVP metabolism in human liver microsomes (NVP 5 µg/ml). A. Standard of NVP (no metabolism); B. NVP after 30 min at 37 °C; C. NVP and NT (10 µg/ml) after 30 min at 37 °C.
Notes
Solutions and materials used for the isolation of rat microsomes have to be kept on ice (4 °C).
Recipes
- 50 mM sodium-potassium phosphate buffer (pH 7.4) [containing 1.15% (wt/vol) KCl, 2 mM EDTA, and 0.25 M sucrose]
Mix 102 mg KH2PO4 with 603 mg Na2HPO4
Add 1.15 g KCl, 74 mg EDTA and 8.5 g sucrose
Add dH2O to 100 ml - 0.1 M potassium phosphate buffer (pH 7.4)
Mix 1.83 g K2HPO4.3H2O with 0.27 g KH2PO4
Add dH2O to 100 ml - Lowry reagent
Mix 49 ml of reagent A with 0.5 ml of reagent B and 0.5 ml of reagent C:
Reagent A: 2% Na2CO3 in 0.1 N NaOH
Reagent B: 1% CuSO4.5H2O in water
Reagent C: 2% potassium sodium tartrate tetrahydrate in water - 50 mM sodium phosphate monobasic buffer (pH 4.6) [containing 0.1% (vol/vol) triethylamine]
Add 7.8 g NaH2PO4.2H2O and 1 ml trimethylamine
Add dH2O to 1 L
Adjust pH to 4.6
Acknowledgments
This protocol was recently described and applied by Usach et al. (2014). Isolation of rat microsomes and composition of mediums used in the inhibition assay were adapted from Kawashima et al. (1999) and Choi et al. (2008), respectively. Extraction of NVP and its metabolites from human microsomes was adapted from Erickson et al. (1999). I. Usach has been granted a predoctoral fellowship from the Atracció de Talent (VLC-CAMPUS) program of University of Valencia.
References
- Choi, Y. H., Chung, S. J. and Lee, M. G. (2008). Pharmacokinetic interaction between DA-8159, a new erectogenic, and metformin in rats: competitive inhibition of metabolism via hepatic CYP3A1/2. Br J Pharmacol 153(7): 1568-1578.
- Erickson, D. A., Mather, G., Trager, W. F., Levy, R. H. and Keirns, J. J. (1999). Characterization of the in vitro biotransformation of the HIV-1 reverse transcriptase inhibitor nevirapine by human hepatic cytochromes P-450. Drug Metab Dispos 27(12): 1488-1495.
- Kawashima, K., Hosoi, K., Naruke, T., Shiba, T., Kitamura, M. and Watabe, T. (1999). Aldehyde oxidase-dependent marked species difference in hepatic metabolism of the sedative-hypnotic, zaleplon, between monkeys and rats. Drug Metab Dispos 27(3): 422-428.
- Lowry, O. H., Rosebrough, N. J., Farr, A. L. and Randall, R. J. (1951). Protein measurement with the Folin phenol reagent. J Biol Chem 193(1): 265-275.
- Usach, I., Melis, V., Gandia, P. and Peris, J. E. (2014). Pharmacokinetic interaction between nevirapine and nortriptyline in rats: inhibition of nevirapine metabolism by nortriptyline. Antimicrob Agents Chemother 58(12): 7041-7048.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Usach, I. and Peris, J. (2015). In vitro Studies: Inhibition of Nevirapine Metabolism by Nortriptyline in Hepatic Microsomes. Bio-protocol 5(19): e1607. DOI: 10.21769/BioProtoc.1607.
Category
Microbiology > Antimicrobial assay > Antiviral assay
Microbiology > Microbial biochemistry > Other compound
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










