- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Adoptive Transfer of Myeloid-Derived Suppressor Cells and T Cells in a Prostate Cancer Model
Published: Vol 5, Iss 16, Aug 20, 2015 DOI: 10.21769/BioProtoc.1557 Views: 9794
Reviewed by: HongLok LungSalma HasanAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Participant-Derived Xenograft Mouse Model to Decode Autologous Mechanisms of HIV Control and Evaluate Immunotherapies
Emma Falling Iversen [...] R. Brad Jones
Apr 5, 2025 2531 Views

PBMC-Humanized Mouse Model for Multiple Sclerosis: Studying Immune Changes and CNS Involvement
Anastasia Dagkonaki [...] Lesley Probert
May 20, 2025 3993 Views
Abstract
The adoptive transfer of immune cells for cancer, chronic infection, and autoimmunity is an emerging field that has shown promise in recent trials. The transgenic adenocarcinoma mouse prostate (TRAMP) is a classical mouse model of prostate cancer (PCa) and TRAMP cell lines were derived from a TRAMP mouse tumor. TRAMP-C2 is tumorigenic when (subcutaneously) s.c. grafted into syngeneic C57BL/6 host mice (Foster et al., 1997). This protocol will describe the adoptive transfer of purified CD11b+Gr1+ double positive (DP) myeloid-derived suppressor cells (MDSC) and CD3+ T cells in the TRAMP-C2 prostate cancer mouse model in order to establish the intrinsic functionality of these immune cells and to determine their role in tumorigenesis in vivo (Yan et al., 2014).
Materials and Reagents
- RPMI 1640 (Life Technologies, Gibco®, catalog number: 22400-089 )
- Trypan blue 0.4% solution (Lonza, catalog number: 17-942E )
- Myeloid-Derived Suppressor Cell Isolation Kit (mouse) (Miltenyi Biotec, catalog number: 130-094-538 )
- Pan T Cell Isolation Kit II, mouse (Miltenyi Biotec, catalog number: 130-095-130 )
- Antibodies for flow cytometry: Ly-6G-FITC (Gr1, RB6-8C5), CD11b-PE (M1/70), and CD3-FITC (17A2) (Biolegend, catalog numbers: 108405 , 01207 , and 100203 respectively)
- MACS® BSA Stock Solution (Miltenyi Biotec, catalog number: 130-091-376 )
- AutoMACS® Rinsing Solution (Miltenyi Biotec, catalog number: 130-091-222 )
- Phosphate buffer saline (PBS) (see Recipes)
- Sterile red blood cell lysis buffer (RBC lysis buffer) (see Recipes)
- MACS buffer (see Recipes)
Equipment
- LS column (Miltenyi Biotec, catalog number: 130-042-401 )
- MidiMACS Separator (Miltenyi Biotec, catalog number: 130-042-302 )
- MACS MultiStand (Miltenyi Biotec, catalog number: 130-042-303 )
- Wide field microscope (Nikon Diaphot Phase Contrast Inverted Laboratory Microscope, catalog number: 805426 )
- Sterile forceps and scissors
- Flow cytometer
- 1 ml syringes (29 G) (BD Biosciences, catalog number: 329410 )
- Sterile Cell strainers 70 μm (BD Biosciences, catalog number: 352350 )
- 15 ml conical tubes (BD Biosciences, catalog number: 352095 )
- Tabletop centifuge
- Cell culture centrifuge
- Sterile culture hood
- Hemocytometer
- 60 mm cell culture dish
Procedure
- Isolation of splenocytes
- Prepare a single cell suspension from mouse (TRAMP-C2 tumor bearing, about 4 months old) spleens in the sterile culture hood. Disrupt the spleen with the plunger of a 1 ml syringe against a 70-μm cell strainer in a 60 mm petri dish filled with 2 ml of RPMI1640.
- Centrifuge single cell suspensions in 15 ml conical tubes at 300 x g for 10 min at RT.
- Re-suspend the splenocytes with 5 ml of RBC lysis buffer and incubate 5 min at RT. Dilute with 10 ml PBS and centrifuge for 10 min at 300 x g. Re-suspend cell pellet in 5 ml MACS buffer (4 °C) and count viable cell numbers using a 0.4% Trypan blue solution. Each spleen yields about 200 x 106 splenocytes.
- One spleen can provide enough Gr1+CD11b+ DP cells for transplantation of 3 experimental mice; the CD3+ cells isolated from one spleen is also enough for transplantation of 3 experimental mice. Splenocytes from 2-3 individual spleens can be pooled before immune cell purification.
- Prepare a single cell suspension from mouse (TRAMP-C2 tumor bearing, about 4 months old) spleens in the sterile culture hood. Disrupt the spleen with the plunger of a 1 ml syringe against a 70-μm cell strainer in a 60 mm petri dish filled with 2 ml of RPMI1640.
- CD11b+Gr1+ DP cells purification from splenocytes using Miltenyi Myeloid-Derived Suppressor Cell Isolation Kit (mouse, a kit for positive isolation of cells)
- Centrifuge cell suspension at 300 x g for 10 min at 4 °C in the 15 ml conical tubes. Aspirate supernatant completely.
- Re-suspend cell pellet in 350 µl of MACS buffer per 108 total cells.
- Add 50 µl of FcR Blocking Reagent per 108 total cells.
- Mix well and incubate for 10 min in the refrigerator (2-8 °C).
- Add 100 µl of Anti-Ly-6G-Biotin (MDSC-Kit).
- Mix well and incubate for 10 min in the refrigerator (2-8 °C).
- Wash cells by adding 10 ml of MACS buffer per 108 cells and centrifuge at 300 x g for 10 min at 4 °C. Aspirate supernatant completely.
- Re-suspend up to 108 cells in 800 µl of MACS buffer.
- Add 200 µl of Anti-Biotin MicroBeads.
- Mix well and incubate for 15 min in the refrigerator (2-8 °C).
- Wash cells by adding 10 ml of MACS buffer per 108 cells and centrifuge at 300 x g for 10 min at 4 °C. Aspirate supernatant completely.
- Re-suspend up to 108 cells in 500 µl of MACS buffer.
- Place the LS column in the magnetic field of a MidiMACS separator.
- Equilibrate the column by rinsing with 3 ml of MACS buffer.
- Apply the cell suspension onto the column; collect flow-through containing unlabeled cells.
- Wash the column with 3 x 3 ml of MACS buffer and collect unlabeled cells that pass through and combine with the effluent from step B15; keep unlabeled cells on ice until further processing.
- Remove the column from the separator and place it in a 15 ml conical tube.
- Pipette 5 ml of MACS buffer onto the column; immediately flush out the magnetically labeled cells by firmly pushing the plunger into the column and collect CD11b+Gr1+ DP cells.
- Count viable cell numbers using a 0.4% Trypan blue solution. Set aside 2 x 105 cells for evaluating purification efficiency as described below.
- Centrifuge cell suspension at 300 x g for 10 min at 4 °C in the 15 ml conical tubes. Aspirate supernatant completely.
- Miltenyi T cells purification from splenocytes using Pan T Cell Isolation Kit II (mouse, a kit for negative isolation of cells)
- Count and centrifuge unlabeled cell suspension from steps B15-16; re-suspend cell pellet in 400 µl MACS buffer per 108 total cells.
- Add 100 μl of Biotin-Antibody Cocktail per 108 total cells.
- Mix well and incubate for 5 min in the refrigerator (2-8 °C).
- Add 300 μl of MACS buffer per 108 total cells.
- Add 20 μl of Anti-Biotin MicroBeads per 108 total cells.
- Mix well and incubate for 10 min in the refrigerator (2-8 °C).
- Place a LS Column in the magnetic field of a MidiMACS Separator.
- Prepare the column by rinsing with 3 ml of MACS buffer.
- Apply cell suspension onto the column and collect flow-through containing unlabeled cells, representing the enriched T cells.
- Wash the column with 3 ml of MACS buffer and collect unlabeled cells that pass through, representing the enriched T cells; combine with the effluent from step C9.
- Count viable cell numbers using 0.4% Trypan Blue solution. Set aside 2 x 105 cells for evaluating purification efficiency as described below.
- Count and centrifuge unlabeled cell suspension from steps B15-16; re-suspend cell pellet in 400 µl MACS buffer per 108 total cells.
- Control of purification efficiency by flow cytometry
- Stain 2 x 105 total cells (step B19) with 20 μl of a suspension contained pre-titrated amounts of anti-mouse Ly-6G and anti-mouse CD11b. The antibodies are 1:100 diluted in PBS with 1% BSA.
- Stain 2 x 105 total cells (step C11) with 20 μl of a suspension contained pre-titrated amounts of anti-mouse CD3.
- Incubate 30 min at 4 °C and wash in 150 μl of washing buffer (PBS with 1% BSA).
- Centrifuge cell suspension at 4 °C and 500 x g for 5 min. Discard supernatant and keep the cell pellet.
- Re-suspend in 200 μl of washing buffer (PBS with 1% BSA) and analyze in a flow cytometer.
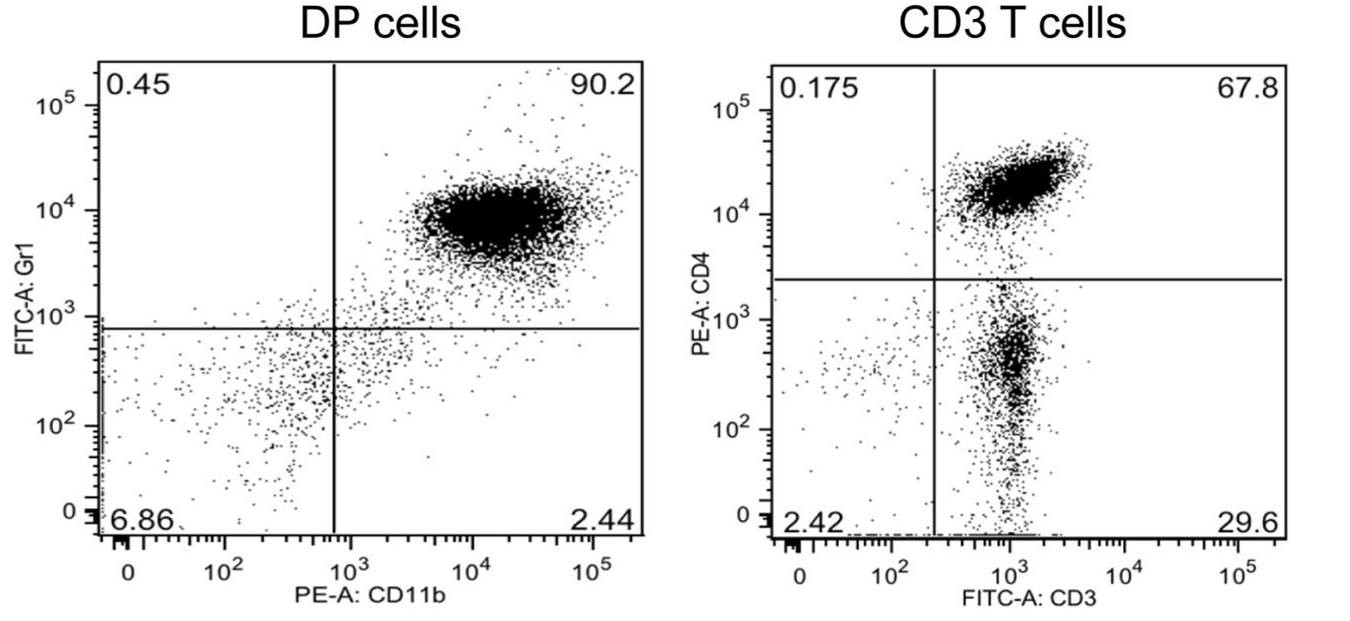
Figure 1. The purity of CD11b+Gr1+ DP cells (Gr1 and CD11b antibodies) and CD3+ T cells (CD4 and CD3 antibodies) can be assessed by FACS analyses - Stain 2 x 105 total cells (step B19) with 20 μl of a suspension contained pre-titrated amounts of anti-mouse Ly-6G and anti-mouse CD11b. The antibodies are 1:100 diluted in PBS with 1% BSA.
- Mouse prostate cancer model and tail vein injection (≥ 5 mice/group)
- Mice with C57/Bl6 background are subcutaneously (s.c.) injected with TRAMP-C2 cells (3 x 106 cells in 0.2 ml PBS per mouse) on the same day of the adoptive transfer of immune cells (Yan et al., 2014). On day 7 and day 14 post-injections, an additional two doses of purified CD11b+Gr1+ DP cells (5 x 106 cells per mouse) or purified CD3+ T cells (5 x 106 cells per mouse) need to be adoptively transferred via intravenous injection. Mice will be sacrificed when they appeared moribund (45 days).
- Warm up the mice under a lamp for 5 min to achieve vasodilation for tail vein injection. Inject purified CD11b+Gr1+ DP cells (5 x 106 cells/200 μl PBS per mouse) or purified CD3+ T cells (5 x 106 cells/200 μl PBS per mouse) in the lateral tail vein with a 1 ml-syringe with a 29 G needle.
- Tumor development will be closely monitored, and tumor size will be measured every 7 days.
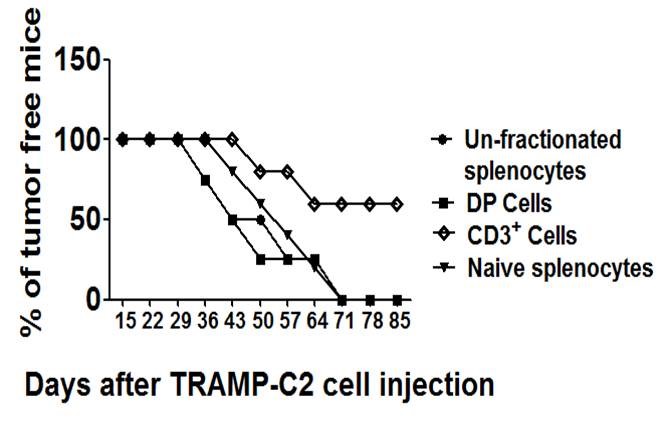
Figure 2. DP, but not T cells from tumor challenged WT mice were sufficient to permit tumor growth in ogr1-/- mice injected with TRAMP0C2 cells. (Yan et al., 2014) - Mice with C57/Bl6 background are subcutaneously (s.c.) injected with TRAMP-C2 cells (3 x 106 cells in 0.2 ml PBS per mouse) on the same day of the adoptive transfer of immune cells (Yan et al., 2014). On day 7 and day 14 post-injections, an additional two doses of purified CD11b+Gr1+ DP cells (5 x 106 cells per mouse) or purified CD3+ T cells (5 x 106 cells per mouse) need to be adoptively transferred via intravenous injection. Mice will be sacrificed when they appeared moribund (45 days).
Recipes
- RBC lysis buffer
0.15 M NH4Cl
1 mM NaHCO3
0.1 mM EDTA dissolved in sterile double distilled water
Adjust pH to 7.2-7.4 with 1 M HCl
Filter sterilize - Phosphate buffer saline (PBS)
136 mM NaCl
8.2 mM Na2HPO4
1.5 mM KH2PO4
2.7 mM KCl (pH 7.4) - MACS buffer
Prepare a solution containing phosphate-buffered saline (PBS) (pH 7.2), 0.5% bovine serum albumin (BSA), and 2 mM EDTA by diluting MACS® BSA Stock Solution1:20 with autoMACS® Rinsing Solution.
Keep buffer cold (2-8 °C).
Degas buffer before use, as air bubbles may block the column.
Acknowledgments
This work was supported in part by the National Institutes of Health (RO1 155145 to YX); and the Mary Fendrich-Hulman Charitable Trust Fund to YX.
References
- Foster, B. A., Gingrich, J. R., Kwon, E. D., Madias, C. and Greenberg, N. M. (1997). Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Cancer Res 57(16): 3325-3330.
- Yan, L., Singh, L. S., Zhang, L. and Xu, Y. (2014). Role of OGR1 in myeloid-derived cells in prostate cancer. Oncogene 33(2): 157-164.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Yan, L. and Xu, Y. (2015). Adoptive Transfer of Myeloid-Derived Suppressor Cells and T Cells in a Prostate Cancer Model. Bio-protocol 5(16): e1557. DOI: 10.21769/BioProtoc.1557.
Category
Cancer Biology > Tumor immunology > Animal models > Cell transfer therapy
Immunology > Animal model > Mouse
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









