- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Bean Pod Mottle Virus (BPMV) Viral Inoculation Procedure in Common Bean (Phaseolus vulgaris L.)
Published: Vol 5, Iss 13, Jul 5, 2015 DOI: 10.21769/BioProtoc.1524 Views: 18464
Reviewed by: Arsalan DaudiShyam SolankiMarisa Rosa

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Quantification of the Composition Dynamics of a Maize Root-associated Simplified Bacterial Community and Evaluation of Its Biological Control Effect
Ben Niu and Roberto Kolter
Jun 20, 2018 10507 Views

Tracking Root Interactions System (TRIS) Experiment and Quality Control
Hassan Massalha [...] Asaph Aharoni
Apr 20, 2019 7349 Views

A Quick Method for Screening Biocontrol Efficacy of Bacterial Isolates against Bacterial Wilt Pathogen Ralstonia solanacearum in Tomato
Heena Agarwal [...] Niraj Agarwala
Nov 20, 2020 5560 Views
Abstract
Viral vectors derived from the Bean pod mottle virus (BPMV) were shown to be highly efficient tools for functional studies in soybean (Glycine max) and common bean (Phaseolus vulgaris) (Zhang et al., 2010; Diaz-Camino et al., 2011; Pflieger et al., 2013; Pflieger et al., 2014). Indeed, BPMV-derived vectors enable successful foreign gene expression analysis as well as virus-induced gene silencing (VIGS) but the delivery procedure of the viral vector into plants (i.e. primary inoculation) is a critical step. It can be achieved by various techniques such as Agrobacterium-mediated infiltration (agro-inoculation), mechanical inoculation of in vitro transcribed RNA, or biolistic delivery of infectious plasmid DNA (i.e. a DNA plasmid carrying a cDNA copy of the modified viral genome under the control of a 35S promoter). These delivery methods may be incompatible with large-scale functional studies (Pflieger et al., 2013). Here, we present the protocol for rapid, cheap and simple mechanical inoculation of BPMV vectors by direct rubbing of infectious plasmid DNA (direct DNA rubbing). Once infected plants are obtained, we used a classical protocol of mechanical inoculation using infected leaf sap to inoculate new healthy common bean plants (i.e. secondary inoculation).
Materials and Reagents
- Phaseolus vulgaris (P. vulgaris) seeds
Notes:- Seeds of common bean genotypes can be obtained from the Centro Internacional de Agricultura Tropical (CIAT, Colombia).
- ‘Black Valentine’ is recommended for primary inoculation to generate infected leaf sap used for further inoculation of any other genotypes. For secondary inoculation, any other genotype of interest can be used.
- Seeds of common bean genotypes can be obtained from the Centro Internacional de Agricultura Tropical (CIAT, Colombia).
- Vermiculite 1-4 mm (Agrena Rungis, catalog number: VERMOYS100 )
- Carborundum 0.037 mm (used as an abrasive) (VWR, catalog number: 22540.298 )
- Recombinant BPMV RNA1 plasmid (pBPMV-IA-R1M) (Zhang et al., 2010) (Note 1)
- Recombinant BPMV RNA2 plasmid (pBPMV-IA-V1, as a BPMV wild-type control) (Zhang et al., 2010) (Note 1)
- Recombinant BPMV RNA2 plasmid (pBPMV-GFP2) (green fluorescence protein) [as a gene expression positive control (Zhang et al., 2010) (Note 1)]
- Recombinant BPMV RNA2 plasmid, (pBPMV-PvPDS391bp) (phytoene desaturase) [as a VIGS positive control (Pflieger et al., 2014) (Note 1)]
- Miracloth (Calbiochem®, catalog number: 475855-1R )
- K2HPO4 (Sigma-Aldrich, catalog number: P3786 )
- KH2PO4 (Sigma-Aldrich, catalog number: P0662 )
- MilliQ water
- Absorbant paper 1500F (Argos, catalog number: 106 )
- Liquid nitrogen
- Qiagen kit “Plasmid Maxi Kit” (QIAGEN, catalog number: 12163 )
- Fertilizer Plant-Prod 14-12-32 (Puteaux SA)
- Fertilizer Fertiligo L (Fertil International SA)
- Nutritive solution (see Recipes)
- 0.1 M potassium phosphate buffer (see Recipes)
- 50 mM potassium phosphate buffer (see Recipes)
Equipment
- Greenhouse or growth chamber for plant growth
- Greenhouse or growth chamber for phytopathological tests (Note 2)
- Pipetmans (Gilson, model: p1000 , p200 , p100 , p20 and p10 ) and tips
- -20 °C refrigerator
- -80 °C refrigerator
- Vortex
- Plastic pots (7 x 7 x 6 cm pots and 20 cm diameter pots of 4 L)
- Growth chamber
- Latex gloves
- Surgical masks
- Microcentrifuge
- Eppendorf microfuge tube
- 1.5 ml microfuge tubes
- Mortar (9 cm diameter) and pestle (12 mm diameter)
- UV lamp for GFP detection (High intensity 100-Watt long-wave UV lamp, UVP®)
- UV face shield (Thermo Fisher Scientific, catalog number: 6355 )
Procedure
- Growth of common bean ‘Black Valentine’ plants for primary inoculation by direct DNA rubbing
- Sow the common bean ‘Black Valentine’ (Note 3) seeds in plastic pots (7 x 7 x 6 cm) filled with moisted vermiculite. Be careful to place the seed just under the vermiculite surface.
- Put the pots in a tub full of tap water and place in a growth chamber at 23 °C under a 16 h light/8 h dark cycle and 75% relative humidity (Note 4).
- Water the seedlings by regularly filling the tub with tap water so that watering is done by capillarity (approximately every two days, in our hands).
- Let the seedlings grow during 10-12 days (the stage at which primary leaves are fully-expanded).
- At this stage of development, the plants are ready to be inoculated (Figure 1A). If you need to maintain the plant for longer than two weeks after inoculation, we recommend to transplant three seedlings in moist vermiculite in a 20 cm diameter pot. Place the pot in a saucer full of water.
- Place the plants in a dark room at 19 °C and 75% relative humidity for 24 h prior to inoculation (Note 4).
- Sow the common bean ‘Black Valentine’ (Note 3) seeds in plastic pots (7 x 7 x 6 cm) filled with moisted vermiculite. Be careful to place the seed just under the vermiculite surface.
- Mechanical inoculation of BPMV vectors by direct DNA rubbing of BPMV-derived infectious plasmids
- For mechanical inoculation of common bean using BPMV-derived infectious plasmids, provide 12 ‘Black Valentine’ plants for each BPMV construct.
- To inoculate one plant, mix 5 μg pBPMV-IA-R1M with 5 μg pBPMV-IA-V1 in a final volume of 20 μl of 50 mM potassium phosphate buffer pH 7 in an Eppendorf microfuge tube (Note 5).
- To inoculate one plant, mix 5 μg pBPMV-IA-R1M with 5 μg pBPMV-GFP2 or gene expression construct plasmid DNA in a final volume of 20 μl of 50 mM potassium phosphate buffer pH 7 in an Eppendorf microfuge tube (Note 5).
- To inoculate one plant, mix 5 μg pBPMV-IA-R1M with 5 μg pBPMV-PvPDS391bp or VIGS construct plasmid DNA in a final volume of 20 μl of 50 mM potassium phosphate buffer pH 7 in an Eppendorf microfuge tube (Note 5).
- Vortex briefly and spin down the tubes in a microcentrifuge.
- Powder the upper surface of one primary leaf per plant with carborundum (for the procedure see Video 1). The approximate amount of dusted carborundum should be as in Video 1 and Figure 1B. Don’t dust too much carborundum because it can generate undesirable necrotic areas after rubbing.
Video 1. BPMV primary inoculation in Phaseolus vulgaris (step B6) - Using a pipetman, put 20 μl DNA plasmid mix on the upper leaf surface at the junction between petiole and blade (Figure 1C).
- Make a rapid spreading of the DNA plasmid drop all over the leaf surface with a gloved finger (Video 2).
Video 2. BPMV primary inoculation in P. vulgaris (steps B8-9) - Rub the leaf surface by making 6 passages with a gloved finger. Make this sequential progress twice (Video 2). All the leaf surface should be rubbed. Be careful not to rub too hard.
- At ~3-4 min after rubbing, rinse the inoculated leaf with tap water contained in a wash bottle until all carborundum is removed (Figure 1D).
- Remove gently the excess of water on the upper side of the leaf using absorbant paper (Figure 1E). As the leaf is weakened, be careful not to tear the leaf (Figure 1F).
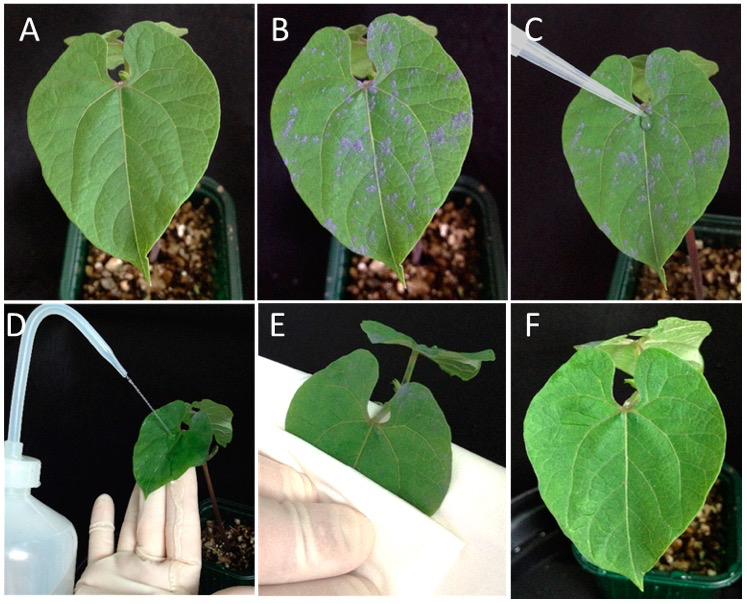
Figure 1. Mechanical inoculation by direct DNA rubbing of BPMV-derived infectious plasmid. A. A fully-expanded primary leaf of ‘Black Valentine’ at 10-12 days. B. One primary leaf per plant powdered with carborundum. C. 20 μl of DNA plasmid mix is put on the upper leaf surface at the junction between petiole and blade. Spreading of the DNA plasmid drop all over the leaf surface is made with a gloved finger as shown in Video 1. D. The inoculated leaf is rinsed intensively with tap water contained in a wash bottle. E. The excess of water is soaked up with absorbant paper. F. The dried inoculated primary leaf. - Place the inoculated plants in a greenhouse or a growth chamber at 19 °C under a 16 h light/8 h dark cycle and 75% relative humidity (Note 4).
- Fertilize plants after mechanical inoculation by pouring the nutritive solution directly in the pot saucer (approximately 500 ml in a 20 cm diameter saucer). Fertilize at a 3-4 days interval.
- Viral symptoms induced by BPMV-Wt occur at about 3 to 4 weeks post-inoculation on the upper systemic leaves that become mottled and bloated (Figure 2A). Successful infection rate using the BPMV-Wt vector is 92-100% (Pflieger et al., 2014).
- Green fluorescence by BPMV-GFP (=pBPMV-IA-R1M + pBPMV-GFP2) can be seen on the primary leaves at about 9 days post-inoculation under UV light and after 17 days post-inoculation on the upper systemic leaves (in Pflieger et al., 2014, see Figure 1). Successful infection rate using the BPMV-GFP vector is 55% (Pflieger et al., 2014).
- White photobleaching phenotype corresponding to PDS gene silencing induced by BPMV-PvPDS391bp (=pBPMV-IA-R1M+pBPMV-PvPDS391bp) is not always observed for primary-inoculated plants (Figure 2B). Successful infection rate using the BPMV- PvPDS391bp vector is 58-91% (Pflieger et al., 2014).
- For each BPMV construct, harvest systemic infected leaflets at 3 to 4 weeks post-inoculation. Preferably choose young upper leaves. Place one leaflet in aluminum paper and freeze the sample in liquid nitrogen.
- Store all the leaflet samples at -80 °C until use for mechanical inoculation of infected leaf tissues (=secondary inoculation).
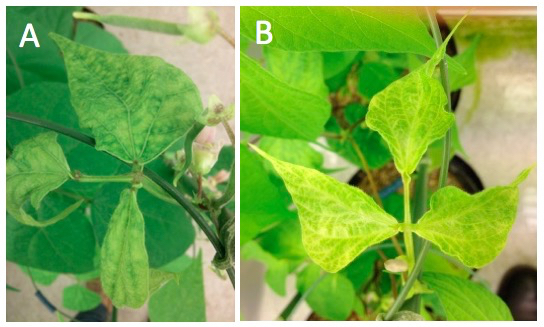
Figure 2. Representative symptoms obtained in P. vulgaris cv. ‘Black Valentine’ plants after mechanical inoculation of BPMV vectors by direct DNA rubbing of BPMV-derived infectious plasmids. A. BPMV-Wt, at 4 weeks post-inoculation. B. BPMV-PvPDS391bp, at 3, 5 weeks post-inoculation.
- For mechanical inoculation of common bean using BPMV-derived infectious plasmids, provide 12 ‘Black Valentine’ plants for each BPMV construct.
- Growth of common bean plants for mechanical inoculation of BPMV vectors using infected leaf tissues (=secondary inoculation)
- Follow the same protocol as described in A (Note 6).
- When other bean genotypes than ‘Black Valentine’ are analyzed, the growth duration of 10-12 days may be delayed until reaching the full-expanded stage of primary leaves.
- Follow the same protocol as described in A (Note 6).
- Mechanical inoculation of BPMV vectors using infected leaf tissues
- Put a fresh or a frozen infected leaflet of common bean ‘Black Valentine’ in a mortar (Figure 3A).
- Grind briefly the tissue with a pestle to obtain a green mash (Figure 3B).
- Add ~3-4 ml of pH7 50 mM potassium phosphate buffer to make leaf sap.
- Grind again with the pestle to obtain a green leaf sap (Figure 3C). As leaf sap is usually heterogeneous, let it settle a few minutes.
- Powder the upper surface of one primary leaf per plant with carborundum (Video 1 and Figure 1A). Don’t dust too much carborundum because it can generate undesirable necrotic areas after rubbing (Note 7).
- Cut a Miracloth piece of ~8 x 6 cm (Figure 3D) and fold it in four (Figure 3E).
- Soak the folded Miracloth in the leaf sap (Figure 3F).
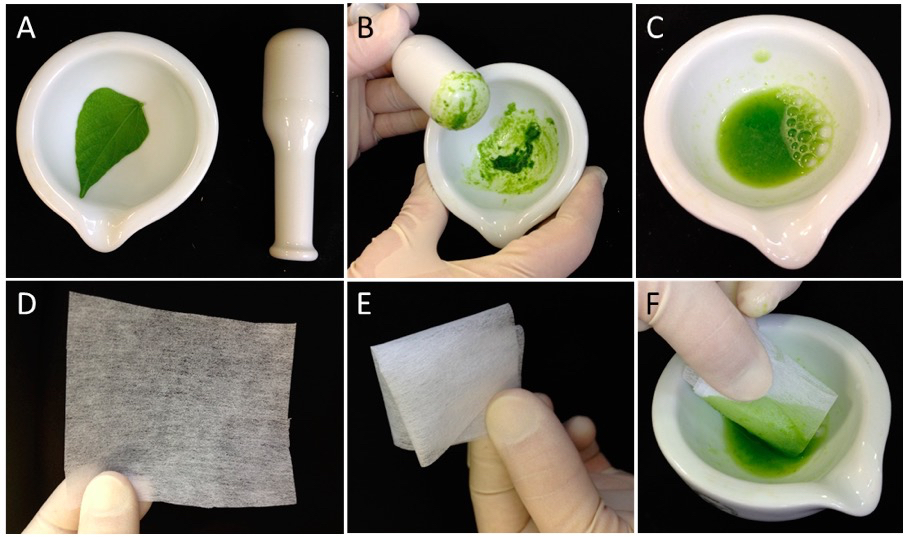
Figure 3. Mechanical inoculation of BPMV vectors using infected leaf tissues. A-C. Preparation of infected leaf sap by grinding an infected leaflet with a mortar and pestle. D-E. The Miracloth piece is folded in four. F. The folded Miracloth piece is soaked in the leaf sap. - Make a rapid rubbing with the soaked Miracloth all over the leaf surface (Video 3).
Video 3. BPMV secondary inoculation in P. vulgaris - Rub the leaf surface by making 6 passages with the folded Miracloth (Video 3). Make this sequential progress only once. All the leaf surface should be rubbed.
- At ~3-4 min after rubbing, rinse the inoculated leaf with tap water contained in a wash bottle until all carborundum is removed (Figure 1D).
- Remove the excess of water using absorbant paper (Figure 1E).
- Place the inoculated plants in a growth chamber or greenhouse at 19 °C under a 16 h light/8 h dark cycle and 75% relative humidity.
- Fertilize plants after mechanical inoculation by pouring the nutritive solution directly in the pot saucer (approximately 500 ml in a 20 cm diameter saucer). Fertilize at a 3-4 days interval.
- Viral symptoms induced by BPMV-Wt occur on the upper systemic leaves at about 2 to 4 weeks post-inoculation depending on the genotype tested (in Pflieger et al., 2014, see Figure 3).
- Green fluorescence by BPMV-GFP (=pBPMV-IA-R1M + pBPMV-GFP2) can be seen on the primary leaves at about 4 to 9 days post-inoculation under UV light and after 10 to 17 days post-inoculation on the upper systemic leaves (in Pflieger et al., 2014, see Figure 1).
- White photobleaching phenotype corresponding to PDS gene silencing induced by BPMV-PvPDS391bp (=pBPMV-IA-R1M + pBPMV-PvPDS391bp) is generally observed at 3 to 4 weeks post-inoculation (in Pflieger et al., 2014, see Figure 6), generally on the young upper leaves.
- For all BPMV vectors, the successful infection rate of secondary inoculation is close to 100% (Pflieger et al., 2014).
- Put a fresh or a frozen infected leaflet of common bean ‘Black Valentine’ in a mortar (Figure 3A).
Notes
- All recombinant plasmids derived from BPMV RNA1 or BPMV RNA2 are carried by recombinant Escherichia coli strains and plasmids were prepared as concentrated ‘maxi-preps’ using the Qiagen kit “Plasmid Maxi Kit” according to the supplier’s instructions. To construct new BPMV vectors containing foreign fragments of interest, refer back to the detailed protocol in Zhang et al. (2013).
- Keep in mind that BPMV is a viral pathogen and that its manipulation must be in accordance with your country legislation in regard to biosafety concern and containment to preclude uncontrolled virus transmission.
- We recommend to use the common bean cv. ‘Black Valentine’ for mechanical inoculation by direct DNA rubbing because this genotype is highly susceptible to BPMV thus producing highly concentrated inoculums for secondary inoculation of any other genotype.
- Phaseolus vulgaris seed germination and growth are optimum at 23 °C. Viral infection and induction of silencing is better at 19 °C. So 24 h prior to inoculation and once inoculated, the plants were placed in a growth room at 19 °C.
- Inoculation of pBPMV-IA-R1M with pBPMV-IA-V1 generates the BPMV-Wt vector, which should be used as a negative control in all experiments. Inoculation of pBPMV-IA-R1M with pBPMV-GFP2 generates the BPMV-GFP vector, which should be used as a positive control in gene expression experiments. Inoculation of pBPMV-IA-R1M with pBPMV-PvPDS391bp generates the BPMV-PDS vector, which should be used as a positive control in VIGS experiments.
- For secondary inoculations using infected leaf tissues, the choice of common bean genotypes may depend of your research goals. Keep in mind that the use of BPMV vectors for gene expression or VIGS requires that the common bean genotype is susceptible to BPMV. You can test this by inoculating your genotype of interest with BPMV-GFP vector. However, in our work we showed that in some susceptible bean genotypes (e.g. JaloEEP558), the GFP fluorescence is only visible in the inoculated primary leaf but the viral vector does not move to the upper non-inoculated leaves and thus no GFP fluorescence is observed in these leaves (Pflieger et al., 2014).
- For secondary inoculations, don’t dust all the plants with carborundum. After rubbing of 20 leaves with the miracloth it will be soaked of leaf sap and carborundum. As an excess of carborundum generates undesirable necrotic areas after rubbing, we recommend not to dust your additional plants with carborundum or really slightly.
Recipes
- Nutritive solution
Fertilizer Plant-Prod 14-12-32 (final concentration = 0.28 kg/L)
Fertilizer Fertiligo L (final concentration = 4.35 ml/L)
Tap water - 0.1 M potassium phosphate buffer (pH 7)
Prepare a solution of KH2PO4 1 M: 27.2 g in 200 ml of MilliQ water
Prepare a solution of K2HPO4 1 M: 34.8 g in 200 ml of MilliQ water
Mix 38.5 ml of KH2PO4 1 M with 61.5 ml of K2HPO4 1 M
Control pH with a pH paper
It should be pH 7 - 50 mM potassium phosphate buffer (pH 7)
25 ml 0.1 M potassium phosphate buffer (pH 7)
25 ml MilliQ water
Acknowledgments
This protocol was developed and optimized for Phaseolus vulgaris by modifying the procedure used for TYMV inoculation in Arabidopsis (Pflieger et al., 2008) and BPMV inoculation in soybean and common bean (Zhang et al., 2010; Zhang et al., 2013). We thank Chunquan Zhang and Steven Whitham at Iowa State University (USA) for sharing the set of BPMV VIGS vectors and providing the common bean cv. ‘Black Valentine’ seeds. CM and MR were supported by fellowships from the French Research Ministry. This work was supported by grants from Institut National de la Recherche Agronomique, Centre National de la Recherche Scientifique, Université Paris-Sud, Saclay Plant Sciences LABEX (SPS), the 3P project (Plant Phenotyping Platform), and the PeaMUST project [grant agreement number ANR-11-BTBR-0002].
References
- Diaz-Camino, C., Annamalai, P., Sanchez, F., Kachroo, A. and Ghabrial, S. A. (2011). An effective virus-based gene silencing method for functional genomics studies in common bean. Plant Methods 7: 16.
- Pflieger, S., Richard, M. M., Blanchet, S., Meziadi, C. and Geffroy, V. (2013). VIGS technology: an attractive tool for functional genomics studies in legumes. Func Plant Biolog 40(12): 1234-1248.
- Pflieger, S., Blanchet, S., Camborde, L., Drugeon, G., Rousseau, A., Noizet, M., Planchais, S. and Jupin, I. (2008). Efficient virus-induced gene silencing in Arabidopsis using a 'one-step' TYMV-derived vector. Plant J 56(4): 678-690.
- Pflieger, S., Blanchet, S., Meziadi, C., Richard, M. M., Thareau, V., Mary, F., Mazoyer, C. and Geffroy, V. (2014). The "one-step" Bean pod mottle virus (BPMV)-derived vector is a functional genomics tool for efficient overexpression of heterologous protein, virus-induced gene silencing and genetic mapping of BPMV R-gene in common bean (Phaseolus vulgaris L.). BMC Plant Biol 14: 232.
- Zhang, C., Bradshaw, J. D., Whitham, S. A. and Hill, J. H. (2010). The development of an efficient multipurpose Bean pod mottle virus viral vector set for foreign gene expression and RNA silencing. Plant Physiol 153(1): 52-65.
- Zhang, C., Whitham, S. A. and Hill, J. H. (2013). Virus-induced gene silencing in soybean and common bean. Methods Mol Biol 975: 149-156.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Pflieger, S., Blanchet, S., Meziadi, C., Richard, M. M. and Geffroy, V. (2015). Bean Pod Mottle Virus (BPMV) Viral Inoculation Procedure in Common Bean (Phaseolus vulgaris L.). Bio-protocol 5(13): e1524. DOI: 10.21769/BioProtoc.1524.
Category
Plant Science > Plant immunity > Disease bioassay
Microbiology > Microbe-host interactions > In vivo model > Plant
Microbiology > Microbe-host interactions > Virus
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











