- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Axenic Cultivation of Mycelium of the Lichenized Fungus, Lobaria pulmonaria (Peltigerales, Ascomycota)
Published: Vol 5, Iss 13, Jul 5, 2015 DOI: 10.21769/BioProtoc.1513 Views: 10675
Reviewed by: Fanglian HeChong HeAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

In Vitro Hyphal Branching Assay Using Rhizophagus irregularis
Takaya Tominaga and Hironori Kaminaka
Aug 20, 2024 2339 Views

Silencing Arbuscular Mycorrhizal Fungal Gene Using Chitosan Nanoparticle-Mediated dsRNA Delivery System
Chumei Yan [...] Xianan Xie
Jun 5, 2025 2645 Views

A Reliable In Planta Inoculation and Antifungal Screening Protocol for Rhizoctonia solani-Induced Sheath Blight in Rice
Alinaj Yasin [...] Palash Deb Nath
Nov 5, 2025 1583 Views
Abstract
Lichens are symbiotic organisms consisting of a fungal partner (the mycobiont) and one or more algal or cyanobacterial partners (the photobiont); moreover lichen thalli comprise a plethora of epi- and endobiotic bacteria and non-lichenized fungi. Genetic markers are the most promising tools for the study of fungal diversity. However, applying genetic methods to intimately admixed symbiotic organisms typically requires the development of species-specific genetic markers, since DNA extraction from environmental specimens implicates the acquirement of total DNA of all symbionts and their cohabitants. While the cultivation of the alga is straight forward, the axenic cultivation of lichen-forming fungi is more difficult due to their very slow growth, as compared with the majority of non-lichenized taxa, and the presence of saprophytic, endophytic and parasitic fungi within the lichen thallus. Moreover, lichen-forming fungi (predominantly ascomycetes, few basidiomycetes) are oligotrophic organisms and thus adapted to nutrient poor conditions; in axenic culture on nutrient-rich media, as normally used for mass production of fast-growing saprophytic fungi, they often autointoxicate. Most lichen-forming fungi are not obligately biotrophic and thus can be cultured in the non-symbiotic state.
Here, we present a protocol for the isolation of the lichen-forming ascomycete Lobaria pulmonaria into axenic culture and for mycelial mass culture as a source of pure fungal DNA. We describe the initiation of axenic cultures on agar plates from germinating ascospores and explain the optimization of the in vitro growth in liquid medium. By grinding the few dense, only centrifugally growing fungal colonies with a homogenizer we obtain lots of smaller, well growing colonies and thus higher amounts of mycelium for DNA or RNA isolation (Honegger and Bartnicki-Garcia, 1991).
Materials and Reagents
- Freshly collected lichen thalli of Lobaria pulmonaria bearing apothecia
- Alpha-cyclodextrin (Sigma-Aldrich, catalog number: C4642 )
- BBLTM cornmeal agar (BD, catalog number: 211132 )
- BactoTM casamino acids (BD, catalog number: 228830 )
- BactoTM malt extract (BD, catalog number: 218630 )
- BactoTM peptone (BD, catalog number: 211677 )
- BactoTM yeast extract (BD, catalog number: 212750 )
- CaCl2.2H2O (Merck, catalog number: 102382 )
- Co(NO3).6H2O (Sigma-Aldrich, catalog number: 60832 )
- CuSO4.5H2O (Sigma-Aldrich, catalog number: RES10395 )
- EDTA (Sigma-Aldrich, catalog number: ED2SS )
- FeSO4.7H2O (Sigma-Aldrich, catalog number: F7002 )
- Glucose (Fluka, catalog number: 49159 )
- H3BO3 (Sigma-Aldrich, catalog number: 31146 )
- KH2PO4 (Merck, catalog number: 1.04873 )
- K2HPO4 (Merck, catalog number: 1.05104 )
- KOH (Fluka, catalog number: 60369 )
- NaCl (Sigma-Aldrich, catalog number: 71376 )
- NaNO3 (Sigma-Aldrich, catalog number: S5506 )
- MgSO4.7H2O (Merck, catalog number: 5856 )
- MnCl2.4H2O (Merck, catalog number: 105927 )
- MoO3 (Sigma-Aldrich, catalog number: M0753 )
- ZnSO4.7H2O (Sigma-Aldrich, catalog number: Z4750 )
- Aluminum foil
- Bidistilled water
- Sterilized tap water
- Liquid nitrogen
- Sterilization agent for hands
- Germination medium (see Recipes)
- Lichen medium (see Recipes)
- Bold’s basal medium (BBM) (see Recipes)
Equipment
- Double edge razor blades (any brand)
- Platinum inoculation needles (flame-resistant)
- Dissection forceps, stainless steel (flame-resistant)
- Spatula, stainless steel (flame-resistant)
- Filter paper circles (ø 45-55 mm) (Schleicher & Schuell 589/2)
- Petri dishes (ø 100 mm, sterile) (plastic, plus 1-2 autoclavable glass dishes) (any brand)
- Perforated bar spoons, ca. 30 cm long, stainless steel (flame-resistant)
- Laboratory bottles (SCHOTT DURAN® 100 ml, 500 ml, and 1,000 ml)
- Erlenmeyer flasks (SCHOTT DURAN® 200 ml, wide neck)
- Plugs for Erlenmeyer flasks, cellulose
- Two glass beakers (500 ml and 200 ml)
- Glass pipets (20 ml, 10 ml, and 1 ml)
- Rubber pipet filler up to 100 ml
- Falcon tubes (50 ml, sterile, nonpyrogenic and DNase-/RNase-free, cryo-resistent) (liquid nitrogen)
- Racks for Falcon tubes (cryo-resistant) (liquid nitrogen)
- Dissecting microscope with an objective lens capable of resolving ascospores (10-30 µm)
- Precision balance with milligram resolution range
- Sterile Bench, ideally with UV radiation
- Bunsen burner
- Autoclave for liquid and solid material
- Homogenizer (either a IKA DI 18 basic or Ultraturrax T 18), mounted on a plate stand with a boss head clamp
- Autoclavable homogenizer rods (made from stainless steel, IKA S 18 N-19G)
- Cryogenic vessel big enough to contain a rack with Falcon tubes
- Growth chamber with (continuous) 15-16 °C±1 °C and 12 h light/12 h dark regime
Note: The humidity must not be controlled but ensure dry conditions within the chamber.
Procedure
- Axenic mycelium
- Collect fresh lichen thalli with mature apothecia (Figure 1A), ideally in autumn to spring, but not during summer because this species does not produce sufficient viable spores during the warm and dry season. Thalli may be stored in glass Petri dishes, air-dry during several days in the refrigerator for up to one week.
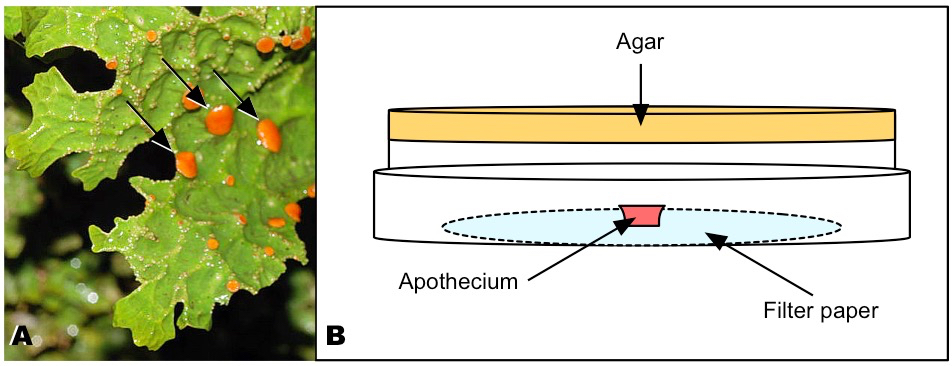
Figure 1. Thallus of Lobaria pulmonaria and arrangement of the apothecium. A. Fresh thallus with apothecia of different ages. Arrows: Mature apothecia suitable for ascospore harvesting; they are convex in the fully hydrated state and approx. 1.5-3.0 mm in diameter. B. Inverted Petri dish with apothecium on moist filter paper. - Thoroughly clean the sterile bench with either 75% alcohol or 5 min UV radiation, or both.
- Prepare the germination medium, autoclave and pour into plastic Petri dishes. Prepare a high number of agar plates for several isolation steps (up to 50 plates). Allow the germination medium to cool down and gelatinize within the sterile bench. With a sterile inoculation needle scratch few superficial lines into the agar (this will help finding the focal plane of the agar surface), then turn the agar plates upside down and continue work.
- Place several filter papers into the glass Petri dish. Wrap Petri dish with aluminum foil and sterilize it in the autoclave. Place one sterile filter paper on the inner surface of the lid of one inverted Petri dish and moisten it with sterile tap water (Figure 1B).
- Use a pointed forceps for excising mature apothecia. Cut the lower part of the apothecia flat by means of a fresh razor blade. Work in sterile bench and place one apothecium on the moisten filter paper by using sterile forceps. The apothecium needs to be fully hydrated, but without superficial water film (this might prevent the ascospores from being ejected). Close the inverted Petri dish, including the apothecium. After having prepared 15-20 Petri dishes, each with one apothecium, incubate the inverted Petri dishes in a growth chamber at 15-16 °C in the dark. The wet paper hydrate the apothecium causing it to discharge ascospores upward onto the medium within minutes or during the first 24 h at the latest.
- Important! After 24 h incubation replace all lids, including filter paper and the apothecium, by new sterile lids and keep the plates at 15-16 °C in the dark.
- Check the Petri dishes every day under the dissecting microscope with transmitted light for bacterial (slimy) or fungal contaminants (see Note 2). Fast-growing fungal contaminants sporulate within a few days and opening the plate will disseminate the wind-dispersed spores. Thus, discard the infected plates without opening!
- Ascospores of L. pulmonaria will germinate within 10-15 days after having been ejected. Place a clean microscope within the sterile bench. Normally, fusiform spores are visible (approx. 18-30 µm long x 5-9 µm wide) (Figure 2A). As these spores are usually three-celled more than one cell may germinate (Figure 2A, asterisk).
- Ignite the Bunsen burner and adjust the blue flame. Thoroughly sterilize the inoculation needles by flaming and allow them to cool down before using. Open a plate with germinated spores under the microscope, pick out one spore using a sterile needle and transfer it onto a fresh Petri dish with germinating medium. Transfer around four spores to each plate, then close the Petri dish and incubate it at 15-16 °C in the dark. Repeat this for each germinating spore.
- Check the plates every second day for the growth rates of the isolates and for contaminations. If single spores are affected by contamination, either cut them out or transfer the others to new agar plates.
- Repeat steps A7-9 during several weeks until the plates contain clean colonies of growing mycelia of L. pulmonaria.
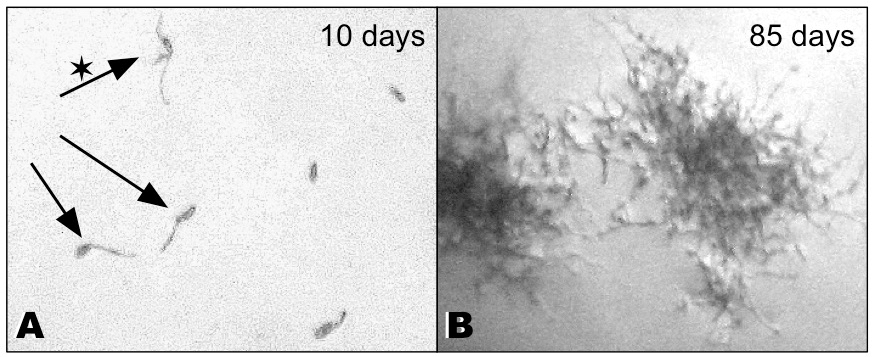
Figure 2. Ascospores and mycelium of L. pulmonaria on agar. A. Germinating ascospores 10 days after spore discharge. Asterisk: Spore with several germ tubes. B. The mycelium of L. pulmonaria grows very slowly. Axenic mycelium 85 days after spore discharge.
- Collect fresh lichen thalli with mature apothecia (Figure 1A), ideally in autumn to spring, but not during summer because this species does not produce sufficient viable spores during the warm and dry season. Thalli may be stored in glass Petri dishes, air-dry during several days in the refrigerator for up to one week.
- Propagation in liquid Lichen Medium
- Prepare 1 L of Lichen Medium and autoclave it with the program for liquids. Wrap cellulose plugs and dissection needles in aluminum foil. Close Erlenmeyer flasks and glass beakers openings with aluminum foil. Autoclave all instruments with the program for solid material.
- Store the Lichen Medium and material in the cleaned sterile bench and wait until they have attained room temperature! Place a microscope into the sterile bench and clean all of its surfaces thoroughly with 75% EtOH.
- Fill 50-100 ml of Lichen Medium into each Erlenmeyer flask. Under the microscope dissect one or several mycelial colonies from the agar surface with a sterile inoculation needle and transfer them to the liquid medium in the Erlenmeyer flask.
- Gently flame the opening of the Erlenmeyer flask, close the flask and keep the aluminum foil on the outer part of the plug intact. Incubate at 15-16 °C with a 12 h/12 h light/dark cycle during a few months, thus allowing the mycelium to grow into a colony visible to the naked eye.
- Wrap each stainless steel rod of the homogenizer with aluminum foil prior to autoclaving; mark the opening of the rod, which will fit to the homogenizer, and keep the blade side covered until it is used. Mount the sterile rod on the homogenizer in a 45° angle and remove the aluminum foil immediately prior to use (Figure 3A). Adjust the homogenizer to level 2 (bigger colonies) or higher (smaller colonies).
- Insert the homogenizer rod into the Erlenmeyer flask with liquid medium and fungal colony. Switch the homogenizer on. Turn the flask slowly during the grinding process, thus making sure that the fungal colonies are getting finely disrupted. This process will kill many cells by disruption, but the majority stays intact and will continue growth. After approx. 10-20 sec homogenization, switch the homogenizer off (Figure 3C). Flame the opening of the Erlenmeyer flask and the cotton plug and seal the flask. Replace the used homogenizer rod by a sterile one for the next flask.
- Incubate at 15-16 °C with 12 h light and 12 h dark during several months, allowing the mycelium pieces to grow visible to the naked eye.
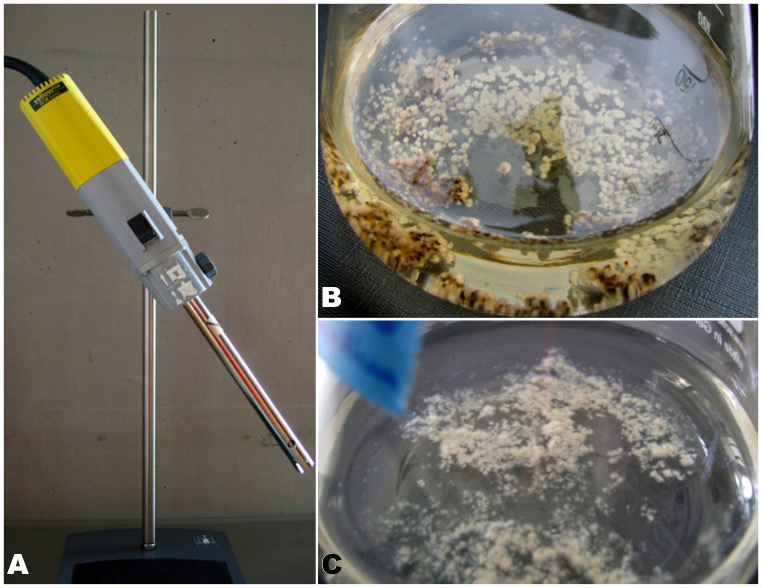
Figure 3. Accelerating fungal growth by homogenization of the mycelium of L. pulmonaria. A. Homogenizer with mounted rod in a 45° angle. B. Erlenmeyer flask containing whitish and brownish mycelium of L. pulmonaria. The dark brown mycelium was overfed on agar plates. It has begun to produce light colored mycelium on the surface of each hyphae clump after transfer into liquid medium (see Note 1). C. Whitish mycelium of L. pulmonaria after homogenization.
- Prepare 1 L of Lichen Medium and autoclave it with the program for liquids. Wrap cellulose plugs and dissection needles in aluminum foil. Close Erlenmeyer flasks and glass beakers openings with aluminum foil. Autoclave all instruments with the program for solid material.
- Harvesting mycelium for DNA or RNA isolation
- Introduce a rack containing a Falcon tube into a cryogenic vessel and fill it with liquid nitrogen. The cryogenic vessel must not be sterile.
- Mount a sterile rod on the homogenizer. Ignite the Bunsen burner. Remove the plug from the Erlenmeyer flask. Harvest some mycelium by using a sterile bar spoon with perforations. Immediately, introduce the mycelium into liquid nitrogen in the Falcon tube by pushing it down from the spoon with a sterile spatula.
- After harvesting, decant the old medium slowly into the 500 ml glass beaker without losing the remaining mycelium and without touching the glass beaker. Refill 50-100 ml fresh medium into the Erlenmeyer flask. Remove the aluminum foil from the homogenizer and grind the mycelium as mentioned above. Flame the Erlenmeyer opening and the cotton plug prior to closing. Repeat steps C1-3 as many times as needed.
- Keep Falcon tubes always filled with liquid nitrogen. Store Falcon tubes at -80 °C. Important: do not completely close the tube; instead, open one twist of the screw thread to let evaporating nitrogen escape.
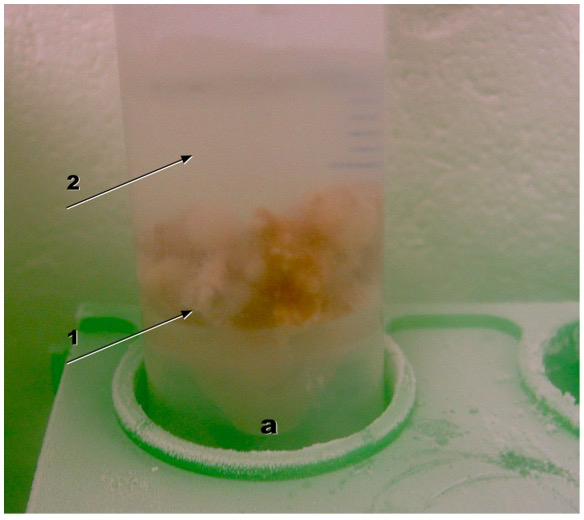
Figure 4. Falcon tube (a) with harvested mycelium (1) in liquid nitrogen (2)
- Introduce a rack containing a Falcon tube into a cryogenic vessel and fill it with liquid nitrogen. The cryogenic vessel must not be sterile.
Notes
- After establishing an axenic mycelium on agar, switch to culture in liquid medium three months after germination at the latest. Do not wait longer! L. pulmonaria is adapted to extremely oligotrophic conditions; thus the mycelium will be overfed on the agar medium and eventually die! Overfed mycelium becomes brownish to dark brown (Figure 3B).
- The decisive criterion to discriminate a fungal contamination from germinating ascospores of L. pulmonaria is the rapidity of growth (Figure 2B; mycelium growth after 85 days). Fungal contaminations produce a dense network of hyphae within a few days and soon start asexual sporulation. Once asexual spores are formed by the contaminant it is almost impossible to rescue the culture, contaminant spores being wind-dispersed already while the lid of the Petri dish is opened.
- To verify the taxonomical belonging of the cultured sample, perform a polymerase chain reaction and sequence the nuclear intragenic transcribed spacer (ITS) rDNA region as described in Cornejo and Scheidegger (2010). The sequence can be checked with BLASTn since the ITS marker of L. pulmonaria is well documented in GenBank.
- The cultures obtained with this method are multispore cultures; in L. pulmonaria, a heterothallic (cross-fertilized) species, each spore might have had different genetic background. If single-spore isolates are required, e.g. for the analysis of the progeny of meiosis, individual spores, as ejected by single asci, can be manually separated and cultured separately (Honegger et al., 2004).
Recipes
- Germination medium (Denison, 2003)
1.0% alpha-cyclodextrin
1.5% cornmeal agar
Preparation of 1 L Germination medium:
Fill in a bottle 15 g cornmeal agar, 10 g cyclodextrin and 1 L bidistilled H2O and autoclave the medium afterwards. The slightly cooled agar can be poured into Petri dishes. - Lichen medium (Honegger, 1985)
BBM 940 ml
Malt extract 0.875 g
Proteose pepton0.125 g
Yeast extract 0.0625 g
Casamino acids0.0625 g
Glucose 0.9375 g - Bold’s basal medium (BBM) (Deason and Bold, 1960)
- Stock solutions
Flask label Compound Dilution of stock solution Dilution of BBM A NaNO3 20 g/800 ml 20 ml B CaCl2 20 g/800 ml 10 ml C MgSO4.7H2O 20 g/800 ml 10 ml D K2HPO4 20 g/800 ml 10 ml E KH2PO4 7 g/400 ml 10 ml F NaCl 1 g/400 ml 10 ml - Trace elements
0.157 g/100 mlNote: *Several elements are mixed in one bottle.Flask label Compound Dilution of trace elements Dilution of BBM g H3BO3 1.142 g/100 ml 1 ml h* FeSO4.7H2O 0.498 g/100 ml 1 ml ZnSO4.7H2O 0.882 g/100 ml MnCl2.4H2O 0.144 g/100 ml i* MoO3 0.071 g/100ml 1 ml CuSO4.5H2O CoNO3.6H2O 0.049 g/100 ml j* EDTA 5.000 g/100 ml 1 ml KOH 3.100 g/100 ml - How to prepare BBM
Mix stock solutions and trace elements (second and third columns). Autoclave stock solutions and trace elements and store them in the refrigerator for future use.
To prepare BBM, mix the amounts of the fourth column and adjust the volume with bidistilled H2O to 1 L. Adjust the pH to 6.0.
- Stock solutions
Acknowledgments
The Swiss National Science Foundation kindly supported this study: projects 31003A-105830 and 31003A-127346 to CS, and projects 3100-064079.00 and 3100A0-103860/1 to RH.
References
- Cornejo, C. and Scheidegger, C. (2010). Lobaria macaronesica sp. nov., and the phylogeny of Lobaria sect. Lobaria (Lobariaceae) in Macaronesia. Bryologist 113: 590-604.
- Deason, T. R. and Bold, H. C. (1960). Phycological studies I. Exploratory studies of Texas soil algae. University of Texas Publications, Nr. 6022, 70.
- Denison, W. C. (2003). Apothecia and ascospores of Lobaria oregana and Lobaria pulmonaria investigated. Mycologia 95(3): 513-518.
- Honegger, R. (1985). The hyphomycetous anamorph of Coniocybe furfuracea. The Lichenologist 17(03): 273-279.
- Honegger, R. and Bartnicki-Garcia, S. (1991). Cell wall structure and composition of cultured mycobionts from the lichens Cladonia macrophylla, Cladonia caespiticia, and Physcia stellaris (Lecanorales, Ascomycetes). Mycological Res 95(8): 905-914.
- Honegger, R., Zippler, U., Gansner, H. and Scherrer, S. (2004). Mating systems in the genus Xanthoria (lichen-forming ascomycetes). Mycol Res 108(Pt 5): 480-488.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Cornejo, C., Scheidegger, C. and Honegger, R. (2015). Axenic Cultivation of Mycelium of the Lichenized Fungus, Lobaria pulmonaria (Peltigerales, Ascomycota). Bio-protocol 5(13): e1513. DOI: 10.21769/BioProtoc.1513.
Category
Microbiology > Microbe-host interactions > Fungus
Microbiology > Microbial cell biology > Cell isolation and culture
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










