- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
An Assay to Test Manganese Tolerance in Arabidopsis
Published: Vol 5, Iss 9, May 5, 2015 DOI: 10.21769/BioProtoc.1460 Views: 9220
Reviewed by: Arsalan DaudiSamik BhattacharyaRenate Weizbauer

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Detection and Quantification of Programmed Cell Death in Chlamydomonas reinhardtii: The Example of S-Nitrosoglutathione
Lou Lambert and Antoine Danon
Aug 5, 2024 1589 Views

Enzymatic Starch Quantification in Developing Flower Primordia of Sweet Cherry
Nestor Santolaria [...] Afif Hedhly
Apr 5, 2025 1897 Views

High-Performance Liquid Chromatography Quantification of Glyphosate, Aminomethylphosphonic Acid, and Ascorbate in Culture Medium and Microalgal Cells
Juan Manuel Ostera [...] Gabriela Malanga
Apr 5, 2025 1182 Views
Abstract
Manganese (Mn) is an essential nutrient required for the catalytic or regulatory function of several cellular enzymes. However, excessive Mn concentrations in plant tissues are toxic to plant cells as they negatively affect enzymatic activities, lead to oxidative stress and disturb the uptake and distribution of other essential mineral elements (Ca, P, Mg or Fe). Plants have developed multiple mechanisms to avoid heavy metals (including Mn) toxicity, including transport across the plasma membrane or tonoplast. The genes encoding transporters involved in Mn detoxification are now being identified in different plant species, and functional characterization of genes isolated from species can be easily carried out in Arabidopsis.
Here we provide a method to evaluate the tolerance to excess Mn of Arabidopsis lines transformed with empty vector pMDC43 or the same vector carrying cucumber gene CsMTP8 encoding putative manganese transporter localized in the vacuolar membrane. We analyzed the growth and developmental phenotypes of plants grown in controlled conditions (phytotrone) on sterile plates containing different concentrations of MnSO4 during a 16 days period. Mn accumulation was measured in the same plants grown in liquid medium supplemented or not (control) with toxic Mn concentration.
Materials and Reagents
- The seeds of Arabidopsis thaliana ecotype Columbia (Col-0) transformed with empty vector (pMDC43) or with vector pMDC43 carrying the coding sequence of CsMTP8 gene under 35S CaM promoter (35S::CsMTP8)
- 96% ethanol
- 5% sodium hypochlorite (NaClO) (commercial detergent ACE produced by Procter and Gamble)
- Sterile water
- 1 M KOH (for pH establishment)
- Murashige & Skoog Basal Medium (MS) (Sigma-Aldrich, catalog number: M5519 )
- Phytagel (Sigma-Aldrich, catalog number: P8169 )
- Salts for media preparation:
Ca(NO3)2 (POCH, catalog number: 874582797 )
MgSO4.7H2O (POCH, catalog number: 613780111 )
KH2PO4 (POCH, catalog number: 742020112 )
K2HPO4 (POCH, catalog number: 742100117 )
HNO3 (POCH, catalog number: 5296041 )
KNO3 (POCH, catalog number: 738910115 )
FeSO4.7H2O (POCH, catalog number: 902840115 )
MnSO4.H2O (POCH, Poland, catalog number: 616940119 )
H3BO3 (POCH, catalog number: 531360115 )
CuSO4.5H2O (POCH, catalog number: ACRS42361 )
ZnSO4.7H2O (POCH, catalog number: Ph. Eur. 6-265762730 )
(NH4)6Mo7O24.4H2O (POCH, catalog number: 139000115 )
Na2EDTA.2H2O (BioShop, catalog number: OM19432 )
- Medium composition for growing plants on plates (see Recipes)
- Medium composition for growing plants in liquid solution (see Recipes)
- 10 mM Na2EDTA (see Recipes)
- 65% HNO3 (see Recipes)
Equipment
- Square (120 x 120 mm) petri dishes polystyrene sterile
- Growth chamber or phytotrone (16/8 h photoperiod at 250 μmol·m−2·s−1 and 23 °C during the day and 22 °C during the night)
- Fume hood
- Laminar flow cabinet
- Autoclave
- Shaker
- Forceps
- Tubes 1.5 ml (Axygen)
- Heating digester with closed vessels (mineralizator) (Kiejdal Digestion Unit DK-20, VELP Scientifica, catalog number: F30100350 )
- Atomic absorption spectrophotometer (Perkin Elmer, model: AAS 3300 )
- Image capturing device (regular digital SLR-single less reflex camera, e.g. Nikon D40 camera)
- Precision balance (± 0.0001)
Procedure
- Sterilization and germination of seeds
- Seeds (10-15 mg) were surface sterilized in 1.5 ml tubes in a laminar flow cabinet by washing in 3% sodium hypochlorite dissolved in 96% ethanol for 5 min, and then by washing five to six times in 96% ethanol. After sterilization, the seeds were dried in laminar for at least two hours.
- Sterilized seeds were sown on Petri dishes (20 seeds per Petri dish: 10 seeds of control seeds with empty vector and 10 seeds carrying CsMTP8) containing 0.5 MS solid medium supplemented or not (control) with 2 mM MnSO4 in laminar flow cabinet (Figure 1A-C). The dishes with seeds were then kept in the dark at 4 °C for 2 days for stratification.
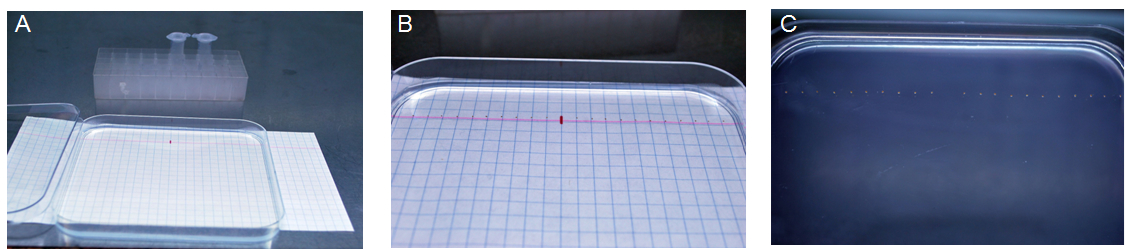
Figure 1. View of seed sowing on petri dishes. The seeds were sterilized in 1.5 ml tubes A. The sterile plate was placed on a squared paper to facilitate the precise sowing and the seeds were placed along the line marked on the paper. B. The space between seeds was 0.5 cm. C. The view of the petri dish after sowing.
- Seeds (10-15 mg) were surface sterilized in 1.5 ml tubes in a laminar flow cabinet by washing in 3% sodium hypochlorite dissolved in 96% ethanol for 5 min, and then by washing five to six times in 96% ethanol. After sterilization, the seeds were dried in laminar for at least two hours.
- Growth tests of A. thaliana plants in manganese excess
- Following stratification, the dishes with seeds were transferred to the culture chamber or phytotrone (22 °C under 16/8 h light/dark photoperiod) and grown for 16 days in vertical position (Figure 2A-B). Then, the plant images were captured using a Nikon D40 camera and the weight of the plants was measured. The experiment was repeated three times with three replicates made for each treatment.
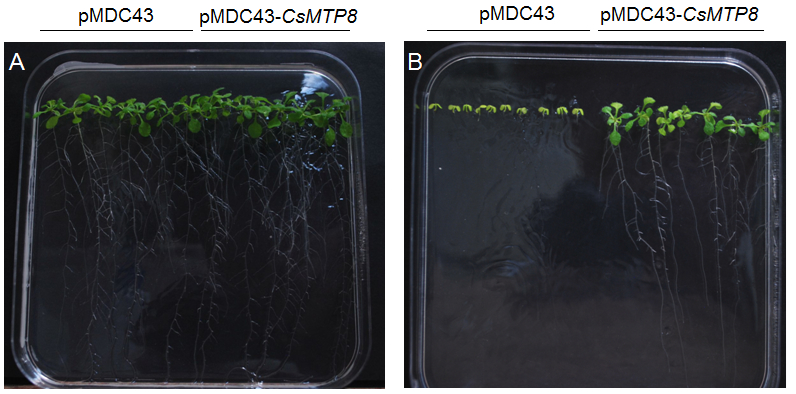
Figure 2. Example of measured effects of Mn excess on Arabidopsis growth in lines transformed with empty vector or vector carrying CsMTP8 (Migocka et al., 2014). Plants were grown in control 0.5 MS (A) or in 0.5 MS supplemented with 2 mM MnSO4 (B) for 16 days in phytotrone (22 °C under 16/8 h light/dark photoperiod).
- Measurement of plant fresh weight. Whole 16 day-old-plants grown in MS medium or MS medium supplemented with 2 mM MnSO4 were collected from a Petri dish using forceps and weighed using a precision balance (Figure 3A-B). Beside the estimation of plant weight, other phenotypes could be assessed in this assay, e.g. root length, leaf number and area or chlorophyll quantity.
3.The obtained data were statistically analyzed using student's t tests and ANOVA (Excel) (P<0.05) (Figure 3C).

Figure 3. The estimation of plant fresh weight. 16-day-old plants were carefully taken from petri dish and weighed with a precision balance (A-B). Data are expressed as means ± standard deviations of three independent experiments with at least 30 plants each (C). Different letters indicate significant differences between control and stress conditions (a) or between the line transformed with empty vector and two lines carrying CsMTP8 (b) (P<0.05; ANOVA Student-t tests).
- Following stratification, the dishes with seeds were transferred to the culture chamber or phytotrone (22 °C under 16/8 h light/dark photoperiod) and grown for 16 days in vertical position (Figure 2A-B). Then, the plant images were captured using a Nikon D40 camera and the weight of the plants was measured. The experiment was repeated three times with three replicates made for each treatment.
- Measurement of manganese accumulation in plants
- To assess Mn accumulation in plants, the 16 day-old-plants grown on petri dishes containing control 0.5 MS were carefully transferred into liquid media of the composition described earlier (Morel et al., 2009), supplemented or not with 50 µM MnSO4 and further grown for five days (Figure 4A-B). Briefly, five plants were gently wrapped with a cotton wool and placed into a 15- ml Falcon containing liquid medium. After five days, plants were carefully harvested, washed in 10 mM Na2EDTA (incubated in a Na2EDTA solution for 5 min on bench, Figure 4C) and dried at 50 °C.

Figure 4. Details of plant preparation for measurement of Mn accumulation. 16-day-old plants were carefully taken from petri dish, wrapped with a cotton wool (A) and transferred into 15-ml Falcon covered with aluminum foil (B; 5 plants per Falcon) containing liquid medium (Morel et al., 2009). After 5 days in phytotrone, the plants were washed in 10 mM Na2EDTA (C) for 5 min and dried.
- The dried plants collected from liquid media supplemented or not with MnSO4 were ground to the powder in mortar with pestle.
- 100 mg of the ground tissue was placed in the glass vessel (Figure 5A) and then digested overnight with 10 ml of the concentrated (65%) HNO3 at room temperature under the fume hood.
- After that, plant samples were boiled in the same solution at 150 °C for 12 h under the fume hood (Figure 5A). The solution was then analyzed by atomic absorbtion spectrophotometry using Perkin Elmer A3300 (Figure 5B). This is a multielement spectrophotometer equipped with cationic lamps (e.g. Mn lamp) which are bought separately. The samples with metal are atomized in a flame and then absorb the specific wavelength emitted by the lamp. However, the elemental analysis by Atomic Absorption Spectrometry provides reliable and reproducible results when the concentration of metal in the samples is relatively high (higher than that met in natural conditions).
- The obtained data were statistically analyzed using student's t tests and ANOVA (Excel) (P<0.05) (Figure 5C).

Figure 5. Details of the analysis of Mn accumulation in Arabidopsis plants. A. Dried plants were digested in closed glass vessels by heating in 10 ml of 65% HNO3 in heating digester at 150 for 12 h. B. Atomic absorbtion spectrophotometer was used to estimate Mn content in the solution following digestion (mineralization). C. The example data are present as means ± standard deviations of three independent experiments with at least 15 plants each. Asterisks indicate significant differences between the line transformed with empty vector and two lines carrying CsMTP8 (b) (P<0.05; ANOVA Student-t tests).
- To assess Mn accumulation in plants, the 16 day-old-plants grown on petri dishes containing control 0.5 MS were carefully transferred into liquid media of the composition described earlier (Morel et al., 2009), supplemented or not with 50 µM MnSO4 and further grown for five days (Figure 4A-B). Briefly, five plants were gently wrapped with a cotton wool and placed into a 15- ml Falcon containing liquid medium. After five days, plants were carefully harvested, washed in 10 mM Na2EDTA (incubated in a Na2EDTA solution for 5 min on bench, Figure 4C) and dried at 50 °C.
Recipes
- Medium composition for growing plants on plates
- For 1 L of 0.5 MS control medium
2.2 g of Murashige & Skoog (MS) medium
Adjust pH to 5.7 with KOH
Add 5 g agar
Sterilized for 20 min at 121 °C/1 atm using an autoclave
- For 1 plate of the medium MS supplemented with 2 mM MnSO4
1 ml of 100 mM MnSO4 solution was added to 50 ml of medium
- For 1 L of 0.5 MS control medium
- Medium composition for growing plants in liquid solution
- For 1 L of control medium
Macroelements:
Microelements:Ca(NO3)2.4H2O
189 mg/L
MgSO4.7H2O
270 mg/L
KH2PO4
100 mg/L
K2HPO4
10 mg/L
KNO3
200 mg/L
FeSO4.7H2O
5.54 mg/L
MnSO4.5H2O
0.59 mg/L
H3BO3
0.56 mg/L
CuSO4.5H2O
0.195 mg/L
ZnSO4.7H2O
0.86 mg/L
(NH4)6Mo7O24.4H2O
0.092 mg/L
Na2EDTA.2H2O
7.44 mg/L
- For 1 L of the medium supplemented with 50 µM MnSO4
0.5 ml of 100 mM MnSO4 solution was added to the medium
- For 1 L of control medium
- 10 mM Na2EDTA
3.722 g disodium ethylenediaminetatraacetate-2H2O (Na2EDTA.2H2O, Mw = 372.24) was added to 800 ml of double distilled H2O and stirred vigorously
The pH was adjusted to 8.0 with NaOH and the volume of the solution was adjusted to 1 L with double distilled water
The solution was sterilized by autoclaving
4.65% HNO3
65% HNO3 was purchased as a ready-to-use concentrated solution
Acknowledgments
We gratefully acknowledge Dr. Sophie Filleur and Dr. Sébastien Thomine (Institut des Sciences du Végétal (ISV), Centre National de la Recherche Scientifique (CNRS), Gif-sur-Yvette, France) for giving us the opportunity to transform of A. thaliana plants. This work was supported by the Polish Ministry of Science and Higher Education (grant no. IP2010 026470). This protocol was adapted from Migocka et al. (2014).
References
- Migocka, M., Papierniak, A., Maciaszczyk-Dziubinska, E., Pozdzik, P., Posyniak, E., Garbiec, A. and Filleur, S. (2014). Cucumber metal transport protein MTP8 confers increased tolerance to manganese when expressed in yeast and Arabidopsis thaliana. J Exp Bot 65(18): 5367-5384.
- Morel, M., Crouzet, J., Gravot, A., Auroy, P., Leonhardt, N., Vavasseur, A. and Richaud, P. (2009). AtHMA3, a P1B-ATPase allowing Cd/Zn/Co/Pb vacuolar storage in Arabidopsis. Plant Physiol 149(2): 894-904.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Migocka, M. and Biskup, R. (2015). An Assay to Test Manganese Tolerance in Arabidopsis. Bio-protocol 5(9): e1460. DOI: 10.21769/BioProtoc.1460.
Category
Plant Science > Plant physiology > Abiotic stress
Plant Science > Plant physiology > Plant growth
Plant Science > Plant biochemistry > Other compound
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









