- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Lignin Extraction and Quantification, a Tool to Monitor Defense Reaction at the Plant Cell Wall Level
Published: Vol 5, Iss 6, Mar 20, 2015 DOI: 10.21769/BioProtoc.1430 Views: 16310
Reviewed by: Ru ZhangAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Isolation of Intact Vacuoles from Arabidopsis Root Protoplasts and Elemental Analysis
Chuanfeng Ju [...] Zhenqian Zhang
Mar 5, 2023 2053 Views

Botrytis cinerea in vivo Inoculation Assays for Early-, Middle- and Late-stage Strawberries
Piao Yang [...] Ye Xia
Oct 20, 2023 2776 Views

High-Performance Liquid Chromatography Quantification of Glyphosate, Aminomethylphosphonic Acid, and Ascorbate in Culture Medium and Microalgal Cells
Juan Manuel Ostera [...] Gabriela Malanga
Apr 5, 2025 1181 Views
Abstract
Lignin is a complex polymer of phenolic compounds (monolignins), which contributes to the rigidity of the plant cell wall. Lignification is essential for plant development, however it is also one of the mechanisms of plant defense. Accumulation of lignin and the polymerization of monolignins at sides of pathogen attack protect the cell wall against cell wall-degrading enzymes and prevent therefore the pathogen’s penetration. In addition to cross-linkage of phenolic compounds, this resistance mechanism includes also callose and cellulose appositions on the cell wall. This results in structures called papillae, which provide the necessary resistance to the mechanical pressure exercised by fungal appressorium. Lignin accumulation in cell walls is therefore a part of plant defense responses. Here we describe a quantification method for lignin and cell wall phenolic compounds, which is based on an acid-catalyzed reaction resulting in a colored and soluble lignin-thioglycolate complex suitable for photometric measurements.
Keywords: Plant DefenseMaterials and Reagents
- Plant material
- Flg22 (a flagellin-derived, 22 amino acid-long peptide) (QRLSTGSRINSAKDDAAGLQIA)
- 80% aqueous methanol (Carl Roth, catalog number: 4627.6 )
- Distilled water
- Acetone (VWR International, catalog number: UN1090 )
- NaOH (Carl Roth, catalog number: 6771.1 )
- 86% H3PO4 (J.T.Baker®, catalog number: 6024 )
- Ethyl acetate (Carl Roth, catalog number: 6784.4 )
- Thioglycolic acid (Sigma-Aldrich, catalog number: T3758 )
- 32% HCl (Carl Roth, catalog number: P074.3 )
- Lignin (alkali) (Sigma-Aldrich, catalog number: 370959 )
- 1 M NaOH (see Recipes)
- 2 M HCl (see Recipes)
Equipment
- Plastic equipment (Grainer Falcon 50 ml tubes, catalog number: 227261 ; Grainer 6-well culture plates, catalog number: 657160 )
- Freeze drying machine
- Tissue Lyser2 (manufactured by Retsch, provided by QIAGEN) and stainless steel beads (QIAGEN, catalog number: 69989 )
- Shaker for tube rotation (with variable temperature)
- Centrifuge (with variable temperature) (Eppendorf, centrifuge number: 5417R )
- Vacuum pump (Savant Systems LLC, catalog number: SC110 : 25-30 Hg)
- Rotary shaker for tube rotation
- Photometer (Eppendorf, BioSpectrometer basic)
Note: The extraction procedure includes a separation of different fractions of phenolic compounds depending on their chemical properties. The soluble phenolic fraction is extracted with 80% methanol (Day 1). The cell wall-bound phenolic fraction is hydrolyzed (alkaline hydrolysis) and solved in ethyl acetate. The lignin fractions are extracted in the later steps of the procedure by binding the to the lingo-thioglycolic acid complex (Days 2 and 3). The soluble and cell wall-bound phenols fractions can be measured according the Folin-Ciocalteau method after Strack et al. (1988), or used for further HPLC analyses. The lignin complex can be measured directly after resolving in NaOH.
Procedure
- Plant material: Arabidopsis seeds were surface-disinfected, germinated, and grown for 2 weeks on ½ MS plant growth media (Murashige and Skoog, 1962). Thereafter, seedlings were transferred into liquid plant growth media for 3 days in 6-well plates for the appropriate treatment. The growth chamber was set to 22 °C with 150 µmol/m2/s light and 8/16 h day/night photoperiod.
- To induce the defense reaction(s), plants were challenged with flg22 for 24 h. Flg22 was added directly to the Arabidopsis seedlings floated on liquid plant growth media, at a final concentration of 100 nM.
- Four grams of Arabidopsis leaves was harvested in 15 ml Falcon tubes and freeze dried (lyophilized) for 3 days.
- Two technical replicates (2 x 40 mg) of Arabidopsis dry leaves were filed into 2 ml tube and homogenized with metal beads in the Tissue Lyser (frequency: 30/sec for 30 sec).
Day 1: Start of extraction
- Before the phenolic extraction, samples were protected against light with aluminum foil.
- One ml of 80% aqueous methanol was added to the samples (plant powder) and incubated for 1 h at room temperature with continuous shaking (120 rpm) to extract the first fraction of soluble phenolic compounds.
- Next, the samples were centrifuged at 13,000 x g for 10 min at 4 °C.
- The supernatants were collected.
- The remaining pellets were again incubated with 1 ml of 80% aqueous methanol for 1 h at room temperature.
- Samples were re-centrifuged at 13,000 x g for 10 min at 4 °C.
- Supernatants were merged and the pellets were used for further extraction.
- Pellets were washed with 1 ml of 80% aqueous methanol, distilled water, and acetone, subsequently.
- The washing steps were performed in each case for 15 min (incubation) followed by centrifugation (13,000 x g for 10 min).
- After the wash with acetone, the pellets were dried in the SpeedVac for 10 min.
- For alkaline hydrolysis, dried pellets were incubated with 1 ml of 1 M NaOH for 1 h at 80 °C and subsequently overnight at room temperature.
Day 2
- The pH was lowered by adding 100 µl of 86% phosphoric acid (H3PO4) to the samples.
- To solve the cell wall-bound phenolic compounds, 500 µl of ethyl acetate was added to the samples and samples were incubated for 30 min at room temperature on a rotary shaker.
- After centrifugation at 13,000 x g for 5 min at room temperature the upper phase (around 500 µl) was collected in new tubes. This supernatant contained the cell wall-bound phenolic compounds.
- Additional 500 µl of ethyl acetate was added to the remaining samples and incubated for 30 min at rotary shaker.
- After centrifugation at 13,000 x g for 5 min at room temperature the upper phase (around 500 µl) was collected and combined with the previous. Both were the cell wall-bound phenolics fraction (about 1 ml).
- For lignin extraction, 500 µl of 80% aqueous methanol was added to the remaining samples (lower phases), and samples were precipitated by centrifugation (13,000 x g for 10 min).
- Resulting pellets were washed with 1 ml 80% methanol, distilled water, and acetone, like in point 13.
- After the wash with acetone, pellets were dried in the SpeedVac for 10 min.
- 1.5 ml of 2 M HCl and 0.3 ml of thioglycolic acid were added to the dry pellets and incubated for 4 h at 95 °C with regular shaking.
- Samples were shortly cooled on ice and centrifuged at 13,000 x g for 5 min at room temperature.
- Supernatants were removed.
- Pellets were washed twice with distilled water and centrifuged at 13,000 x g for 10 min at room temperature.
- The residues were incubated with 1 ml of 0.5 M NaOH overnight on a shaker.
Day 3
- After the overnight incubation samples were centrifuged at 13,000 x g for 5 min at room temperature.
- The supernatants were collected and stored in new 2 ml tubes.
- 0.5 ml NaOH was again added to the remaining pellets and centrifuged at 13,000 x g for 5 min at room temperature.
- The supernatants were combined and acidified with 300 µl of 32% HCl, for the ligno-thioglycolic acid complex precipitation.
- Samples were incubated for 4 h at 4 °C with continuous shaking.
- Thereafter, samples were centrifuged at 13,000 x g for 5 min at room temperature.
- The supernatant was discarded and the lignin pellet was solved in 600 µl of 0.5 M NaOH.
- The quantification of lignin was performed after appropriate dilution (if necessary) by measuring the absorbance at 340 nm. Calibration was performed using a standard curve made of lignin, alkali (Figure 1).
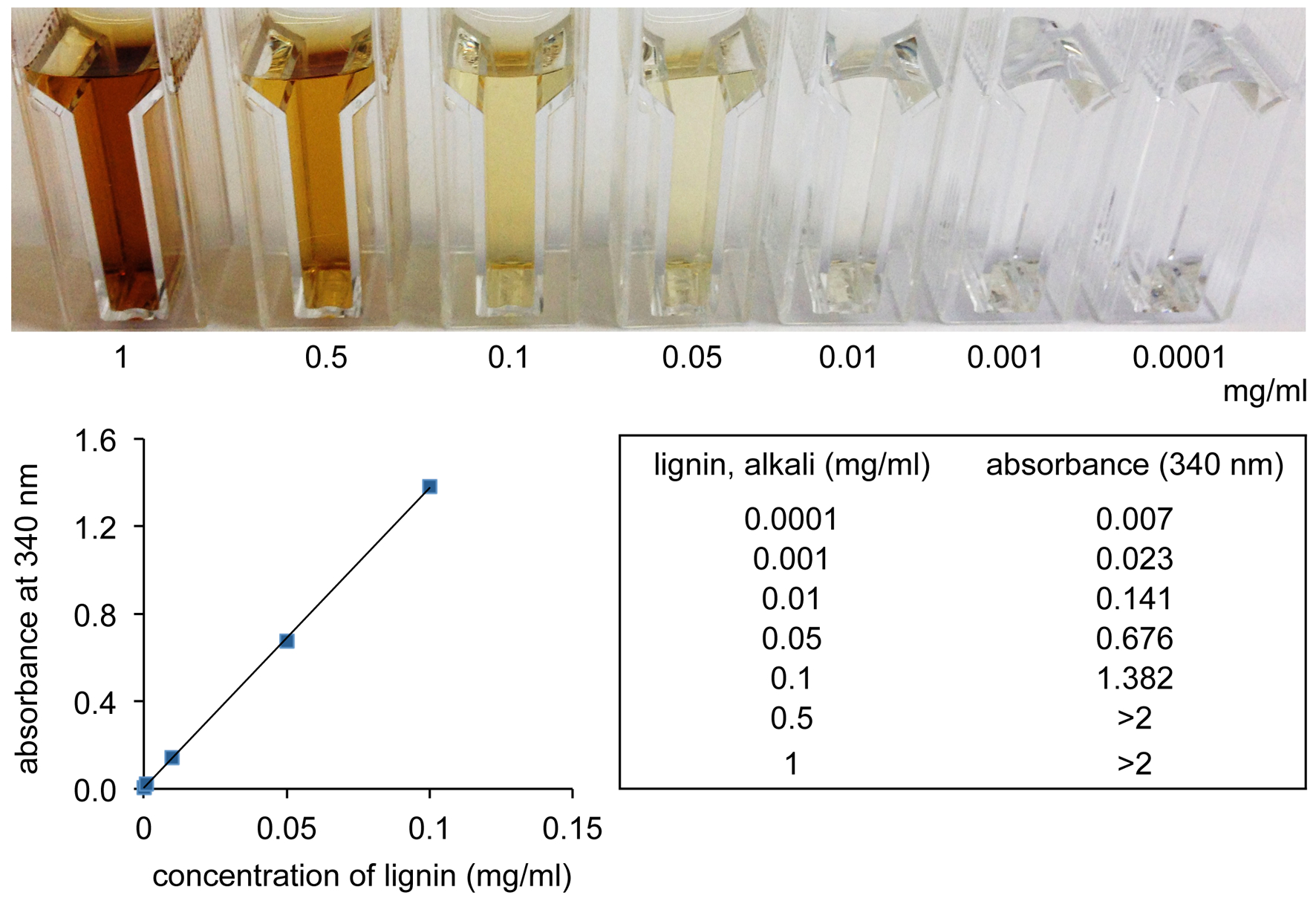
Figure 1. Standard curve of lignin. The colorimetric visualization of lignin was performed in a concentration range of 1 to 0.0001 mg/ml. Lignin was solved in 0.5 M NaOH. The absorbance at 340 nm was measured with a bio-spectrometer and the regression line was calculated accordingly.
Representative data
- For representative data see Schenk et al. (2014).
- For reproducibility see Notes.
Notes
Working under a laboratory fume hood during the whole extraction procedure is recommended. The metal beads used to crush the plant material should be removed in the first steps of extraction procedure. For reproducibility dry plant material should be weighed for adequate calculation, the content of roots and leave tissue should be the same in all samples, and the dry plant material should be homogenized via TissueLyser to an appropriated powder.
Recipes
- 1 M NaOH
2 g of NaOH in 50 ml H2O
- 2 M HCl
1.964 ml of 32% HCl in 10 ml H2O
Acknowledgments
The protocol was modified after Bruce and West (1989), Eynck et al. (2009) and Strack et al. (1988). This work was supported by the Federal Office for Agriculture and Food (BLE), Germany.
References
- Bruce, R. J. and West, C. A. (1989). Elicitation of lignin biosynthesis and isoperoxidase activity by pectic fragments in suspension cultures of castor bean. Plant Physiol 91(3): 889-897.
- Eynck, C., Koopmann, B., Karlovsky, P. and von Tiedemann, A. (2009). Internal resistance in winter oilseed rape inhibits systemic spread of the vascular pathogen Verticillium longisporum. Phytopathology 99(7): 802-811.
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plantarum 15, 473-497.
- Schenk, S. T., Hernandez-Reyes, C., Samans, B., Stein, E., Neumann, C., Schikora, M., Reichelt, M., Mithofer, A., Becker, A., Kogel, K. H. and Schikora, A. (2014). N-Acyl-homoserine lactone primes plants for cell wall reinforcement and induces resistance to bacterial pathogens via the salicylic acid/oxylipin pathway. Plant Cell 26(6): 2708-2723.
- Strack, D., Heilemann, J., Momken, M., and Wray, V. (1988). Cell wall-conjugated phenolics from coniferae leaves. Phytochemistry 27, 3517-3521.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Schenk, S. T. and Schikora, A. (2015). Lignin Extraction and Quantification, a Tool to Monitor Defense Reaction at the Plant Cell Wall Level. Bio-protocol 5(6): e1430. DOI: 10.21769/BioProtoc.1430.
Category
Plant Science > Plant immunity > Disease bioassay
Plant Science > Plant cell biology > Cell staining
Plant Science > Plant biochemistry > Other compound
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









