- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Creating a Rat Model of Chronic Variate Stress
Published: Vol 4, Iss 23, Dec 5, 2014 DOI: 10.21769/BioProtoc.1315 Views: 11875
Reviewed by: Soyun KimEmmanuelle BerretAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Evaluating Working Memory on a T-maze in Male Rats
Ahmed M. Hussein [...] Volker Korz
Jul 20, 2018 10280 Views

Classic Labyrinth Test for Neurobehavioral Evaluation in Wistar Rats
Salim Gasmi
Sep 20, 2018 6880 Views

Consummatory Successive Negative Contrast in Rats
Ana María Jiménez-García [...] Ignacio Morón
Apr 5, 2019 5054 Views
Abstract
Stress is a condition of human experience and an important factor in the onset of various diseases. There are numerous studies showing how stress can accelerate cell aging, immune senescence and some age-related diseases such as neurodegenerative disorders and osteoporosis. However, the effects of stress have different consequences depending on the type, duration or severity and predictability of the stressor applied. Although stress can be beneficial in its acute phase, repeated and severe stressful stimuli produce adverse effects. There are different models of stress depending on the exposure time; acute (when the stressor is applied for a short time, e.g. hours or days, and intensely) or chronic (when the stressor is applied for a long time, e.g. weeks or months, and less intensely. In these cases, the stressor can be repeated each time or different stressors can be used). The latter model is most frequently used to achieve similar conditions to those found in human diseases related to stress. Also, there are several different paradigms depending on the purpose of the study [development of drug therapies or modeling depressive behaviors; for the different paradigms see Dagnino-Subiabre, (2012)]. Here, we describe a 9-day variable-stressor paradigm with repeated and prolonged stimulation and a random daily stressor over days or weeks to minimize its predictability. This protocol has been adapted from other models of variable stress with significant modifications. The absence of predictability of the stressor applied is an important characteristic of this model compared to other models in which repeated stress is used. We avoid the use of a strong stressor, such as foot shock or tail pinch, and describe an easily reproducible new chronic mild stress model. Some models of chronic mild stress have been reported to lead to a wide range of behavioral disturbances and have been proposed as models of depression in animal studies (Cryan et al., 2005).
Keywords: StressMaterials and Reagents
- Male albino Wistar rats (250-270 g)
Equipment
- Glass tank (44 x 33 x 30 cm)
- Plastic tube (21 x 6 cm, 6 cm diameter)
- Individual cages (47 x 32 x 20 cm)
- Cold room or refrigerator (4 °C)
Procedure
The stressors schedule used in this protocol is listed in Table 1. Application of stress starts at a different time every day to minimize its predictability (from 8:00 a.m. to 8:00 p.m.).
Table 1. Schedule of stressors used during chronic variant stress treatment
----------------------------------------------
Day Stressor Time
----- ----------------------- ------------
1 Forced swimming 10 min
2 Restraint 3 h
3 Water deprivation 24 h
4 Restrain at 4 °C 90 min
5 Isolation 24 h
6 Food deprivation 24 h
7 Water deprivation 24 h
8 Restrain at 4 °C 2 h
9 Food deprivation 24 h
-----------------------------------------------
Day 1: Forced swimming
- Fill a glass tank 22 cm deep with water at 23 ± 2 °C (Figure 1).
- Place the animal in the glass tank for 10 min. While the animal is swimming it may try to get out of the tank, so to prevent escape it may be necessary to carefully close it with a heavy lid.
- Return the animal to a clean and dry cage with fresh bedding in order to avoid chills and colds.

Figure 1. Picture of a typical glass tank filled with cold water
Day 2: Restraint
- Place the animal in a 21 x 6 cm plastic tube; adjust it with plastic tape on the outside so the animal is unable to move (Figure 2). The tube must have a 6 cm hole at the far end to allow regular breathing. To place the animal in the plastic tube, it is necessary to place the head of the animal close to the entrance, after which it should enter by itself (Figure 3).

Figure 2. Picture of the tube with tape and animal inside

Figure 3. Pictures showing the restraint procedure using a plastic tube
- Wait for 3 h. Although the plastic tube should be sufficient to prevent the animal coming out is desirable to leave the immobilizer inside the cage.
- Return the animal to its cage. The best way to extract the animal is to make a sudden movement downwards, dropping it into the cage. Try to avoid pulling from the tail.
Day 3: Water deprivation
- Remove the bottle of water from the cage during 24 h. If the animal house is supervised by staff, indicate that the water/food must not be replaced in that cage.
- Place the bottle of water back after the time point is reached.
Day 4: Restraint at 4 °C
- Place the animal in the above-mentioned plastic tube.
- Place the tube with the animal inside a cold room or a refrigerator at 4 °C for 90 min. Cold is a well-documented stressor (Krishnamurthy et al., 2011). Therefore, it is necessary to find a place with these features to put the animals in.
- Return the animal to its cage at room temperature.
Day 5: Isolation
- Place the animal alone in a new cage.
- Return the animal to the cage with their mates.
Day 6: Food deprivation
- Remove the food from the cage for 24 h.
- Place the food back.
Day 7: Water deprivation
- Remove the water container from the cage for 24 h.
- Place the bottle of water back.
Day 8: Restraint at 4 °C
- Put the animal in the plastic tube described above.
- Place the tube with the animal inside a cold room or a refrigerator at 4 °C for 2 h. It has been observed that after the first cold-environment exposure, 90 min at 4 °C is not enough time any more to reach adequate stress levels, that is why 2 h at 4 °C is more convenient.
- Return the animal to its cage at room temperature.
Day 9: Food deprivation
- Remove the food from the cage for 24 h.
- Place the food back.
Representative data
Validation of the stress model: Changes in body and adrenal glands weight, in the blood levels of corticosterone, and in dopamine (DA) and 3,4-Dihydroxyphenylacetic acid (DOPAC) levels in the prefrontal cortex are typical effect of stress and are used as methods to assess stress models. With this chronic variant stress protocol, the body weight of the animals decreases, whereas adrenal weight and the blood levels of corticosterone increase (Figure 4). It has also been found that increased levels of dopamine and DOPAC in the prefrontal cortex are observed (Table 2).
Notes about reproducibility and variability: Stress perception is very subjective and each animal, just as each person, is stressed to a different degree. Therefore, it is normal that the results present some variability. To avoid this, glucose preference test is recommended (Hu et al., 2010); then, only animals that have been actually stressed must be included in the study.
Table 2. DA and DOPAC amount in brain cortex in control and stressed rats
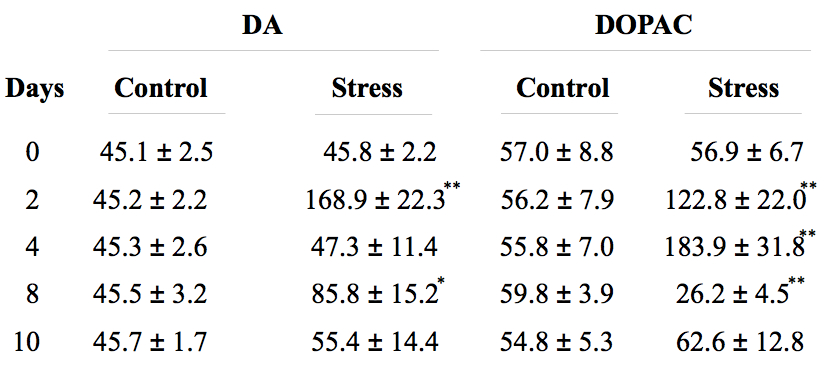
Animals were killed at different time points after treatment (0, 2, 4, 8, and 10 days respectively), and the prefrontal cortex was harvested and processed for DA and DOPAC quantification by HPLC as described in de Pablos et al. (2006). Numbers are expressed as nanograms per gram of wet tissue and are Mean ± SD of five independent experiments. *p<0.05, **p<0.01, statistical significance (Student’s t test) compared with control animals.
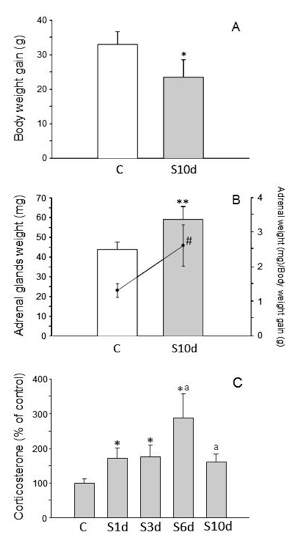
Figure 4. A. Body weight gain (g); B. Adrenal glands weight (mg, bars) and ratio between adrenal glands weight and body weight gain (scatter plot and line). C. Serum corticosterone (percentage of control animals). Statistical signification: Student’s t test comparing before C and after 10 days of variate stress (S10d); *, p< 0.05; **, p< 0.01; #, p< 0.01 (for the ratio adrenal glands weight/body weight gain). One-way ANOVA followed by the LSD post hoc test for multiple range comparisons, p< 0.01; *, compared with the control; a, compared with the previous time point (S1d to S10d indicate the days subjected to variate stress).
Acknowledgments
Chronic-variable stress was adapted from other models of variable stress (Gamaro et al., 2003; Konarska et al., 1990; Murua and Molina, 1992; Muscat et al., 1992; Papp et al., 1991; Willner et al., 1987) with significant modifications. This work was supported by grant SAF-2012-39029 from the Spanish Ministry of Economy and Competitiveness and P10-CTS-6494 (Proyecto de Excelencia of Junta de Andalucia).
References
- Cryan, J. F. and Holmes, A. (2005). The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov 4(9): 775-790.
- Dagnini-Subiabre, A. (2012). Modelos animales para el estudio del estrés y las conductas depresivas. Rev Farmacol Chile 5(1):19.
- de Pablos, R. M., Herrera, A. J., Espinosa-Oliva, A. M., Sarmiento, M., Munoz, M. F., Machado, A. and Venero, J. L. (2014). Chronic stress enhances microglia activation and exacerbates death of nigral dopaminergic neurons under conditions of inflammation. J Neuroinflammation 11: 34.
- de Pablos, R. M., Villaran, R. F., Arguelles, S., Herrera, A. J., Venero, J. L., Ayala, A., Cano, J. and Machado, A. (2006). Stress increases vulnerability to inflammation in the rat prefrontal cortex. J Neurosci 26(21): 5709-5719.
- Gamaro, G. D., Manoli, L. P., Torres, I. L., Silveira, R. and Dalmaz, C. (2003). Effects of chronic variate stress on feeding behavior and on monoamine levels in different rat brain structures. Neurochem Int 42(2): 107-114.
- Hu, H., Su, L., Xu, Y. Q., Zhang, H. and Wang, L. W. (2010). Behavioral and [F-18] fluorodeoxyglucose micro positron emission tomography imaging study in a rat chronic mild stress model of depression. Neuroscience 169(1): 171-181.
- Konarska, M., Stewart, R. E. and McCarty, R. (1990). Predictability of chronic intermittent stress: effects on sympathetic-adrenal medullary responses of laboratory rats. Behav Neural Biol 53(2): 231-243.
- Krishnamurthy, S., Garabadu, D., Reddy, N. R. and Joy, K. P. (2011). Risperidone in ultra low dose protects against stress in the rodent cold restraint model by modulating stress pathways. Neurochem Res 36(10): 1750-1758.
- Murua, V. S. and Molina, V. A. (1992). Effects of chronic variable stress and antidepressant drugs on behavioral inactivity during an uncontrollable stress: interaction between both treatments. Behav Neural Biol 57(1): 87-89.
- Muscat, R., Papp, M. and Willner, P. (1992). Reversal of stress-induced anhedonia by the atypical antidepressants, fluoxetine and maprotiline. Psychopharmacology (Berl) 109(4): 433-438.
- Papp, M., Willner, P. and Muscat, R. (1991). An animal model of anhedonia: attenuation of sucrose consumption and place preference conditioning by chronic unpredictable mild stress. Psychopharmacology (Berl) 104(2): 255-259.
- Willner, P., Towell, A., Sampson, D., Sophokleous, S. and Muscat, R. (1987). Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 93(3): 358-364.
Article Information
Copyright
© 2014 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
de Pablos, R. M., Sarmiento, M. and Espinosa-Oliva, A. M. (2014). Creating a Rat Model of Chronic Variate Stress. Bio-protocol 4(23): e1315. DOI: 10.21769/BioProtoc.1315.
Category
Neuroscience > Behavioral neuroscience > Animal model > Rat
Neuroscience > Nervous system disorders > Animal model
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










