- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Visualization of Cell Complexity in the Filamentous Cyanobacterium Mastigocladus laminosus by Transmission Electron Microscopy (TEM)
Published: Vol 4, Iss 23, Dec 5, 2014 DOI: 10.21769/BioProtoc.1305 Views: 11117
Reviewed by: Fanglian HeClaudia Catalanotti

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Cryo-transmission Electron Microscopy of Outer-inner Membrane Vesicles Naturally Secreted by Gram-negative Pathogenic Bacteria
Lidia Delgado [...] Elena Mercadé
Sep 20, 2019 6747 Views

Rapid Detection of Proliferative Bacteria by Electrical Stimulation
Conor LA Edwards [...] Munehiro Asally
Feb 5, 2020 6565 Views

Tracking the Subcellular Localization of Surface Proteins in Staphylococcus aureus by Immunofluorescence Microscopy
Salvatore J. Scaffidi [...] Wenqi Yu
May 20, 2021 7812 Views
Abstract
The cyanobacterium Mastigocladus laminosus (M. laminosus) is one of the most morphologically complex prokaryotes. It forms long chains of cells that are connected via septal junction complexes; such complexes allow diffusion of metabolites and regulators between neighboring cells. Cellular division occurs in multiple planes, resulting in the formation of true branches, and cell differentiation leads to the formation of specialized cell types for nitrogen fixation (heterocysts) and culture dispersal (hormogonia and necridia). Here, we describe a detailed protocol for the preparation of M. laminosus for TEM in order to visualize the ultrastructural properties of the organism. The presented preparation method is based on adding potassium permanganate as fixative which has been shown to increases the contrast of membranes (Luft, 1956), making it suitable for studies in cyanobacteria where the visualization of the photosynthetic membranes is important.
Keywords: UltrastructureMaterials and Reagents
- Liquid culture of Mastigocladus laminosus
- Potassium phosphate monobasic (KH2PO4) (Thermo Fisher Scientific, catalog number: BP362 )
- Sodium phosphate dibasic (Na2HPO4) (Thermo Fisher Scientific, Acros Organics, catalog number: 204855000 )
- Glutaraldehyde (25%, EM grade) (Agar Scientific, catalog number: R1020 )
- Low gelling temperature agarose (Sigma-Aldrich, catalog number: A9414 )
- Potassium permanganate (KMnO4) (VWR International, BDH, catalog number: 296444N )
- 100% ethanol
- Propylene oxide (Agar Scientific, catalog number: R1080 )
- Araldite CY212 (Agar Scientific, catalog number: R1042 )
- Methyl nadic anhydride (MNA) (Agar Scientific, catalog number: R1083 )
- Dodecenylsuccinic anhydride (DDSA) (Agar Scientific, catalog number: R1052 )
- Benzyldimethylamine (BDMA) (Agar Scientific, catalog number: R1061 )
- Sodium tetraborate (borax) (Sigma-Aldrich, catalog number: 221732 )
- Toluidine blue (Sigma-Aldrich, catalog number: 89640 )
- Uranyl acetate [UO2(CH3COO)2]*2 H2O] (SPI Supplies, catalog number: 02624-AB )
- Lead (II) nitrate [Pb(NO3)2] (Sigma-Aldrich, catalog number: 228621 )
- Tri-sodium citrate (TAAB, catalog number: S011 )
- Sodium hydroxide (NaOH) (Sigma-Aldrich, catalog number: 221465 )
- 0.125 M Sørensen's phosphate buffer (PB) (see Recipes)
- 4% (v/v) glutaraldehyde in PB (see Recipes)
- 2% (w/v) low gelling temperature agarose (see Recipes)
- 2% (w/v) potassium permanganate (see Recipes)
- Araldite (see Recipes)
- 1% (w/v) toluidine blue (see Recipes)
- Uranyl acetate (see Recipes)
- Reynold's lead citrate stain (see Recipes)
Equipment
- Centrifuge
- Copper grids (300 mesh) (Agar Scientific, catalog number: G2740C )
- Dental wax
- Disposable plastic Pasteur pipettes
- Disposable polyethylene beaker
- Eppendorf tubes (1.5 ml)
- Glass or diamond knife
- Hotplate
- Light microscope
- Oven (60 °C)
- Razor blades
- Rotator
- Rubber embedding mould
- Sealable glass vials (7 ml)
- Shaker
- Spatula
- Sterilization filters (pore size: 0.22 µm)
- Syringe
- TEM (JOEL, model: JEM-1230 )
- Tweezers
- Ultramicrotome (Reichert Ultracut E)
- Vortexer
- SPI Slide-A-GridTM storage box (SPI Slide-A-GridTM, catalog number: 02450-AB )
Procedure
Note: Some chemicals used in this protocol are highly toxic and should be used only under the fume hood with adequate precautions. Please check the MSDS before using them.
Preparation of samples for ultra-thin section transmission electron microscopy requires multiple days. The timeline for the different phases of preparation is shown in Table 1.
Table 1. Timeline for preparation of samples for transmission electron microscopy
Days 1-7 Growth of M. laminosus (A)
Day 8 Fixation and pre-embedding (B); Storage for up to 3 months possible
Days 9-11 Dehydration and embedding in Araldite (C); Indefinite storage possible
Day 12 Sectioning and post-staining (D); Storage for up to 1 year possible
Day 13 Visualization at the transmission electron microscope
- Growth of M. laminosus
M. laminosus was grown in gas washing bottles filled with Castenholz D or ND medium (lacking combined nitrogen) (Castenholz, 1988) by bubbling with sterile air. Cultures were kept at 40 °C under constant white light at approximately 20 µE/m2/s until a dense cell mat was visible at the surface (Figure 1).
Figure 1. Dense cell mat of M. laminosus grown in Castenholz D medium - Fixation and pre-embedding
- Transfer a piece of the cell mat (corresponding to a volume of approximately 100 µl) into an Eppendorf tube using a 1 ml pipette.
- Add 1 ml of PB, centrifuge for 2 min at 3,000 x g and remove supernatant.
- Resuspend the cells in 500 µl of 4% (v/v) glutaraldehyde (toxic!) and leave the tube standing for 2 h at room temperature.
- Centrifuge cells for 2 min at 3,000 x g and remove supernatant. Wash cells 3x in 1 ml of PB with 10 min of incubation time between each step to remove the fixative. Resuspend cells by inverting the tube.
- Remove supernatant after the final centrifugation step.
- Resuspend cells in an approximately equal volume of 2% (w/v) liquid agarose (place vial at 60 °C ca. 30 min prior usage to melt the agarose and keep it fluid until usage). Leave sample at room temperature until it is fully polymerized. It is possible to stop the protocol at this step. Samples can be stored in the fridge for up to 3 months.
- Cut the cell pellet with a razor blade into small cubes of about 1-2 mm length.
- Transfer cubes to a glass vial and add 2% (w/v) potassium permanganate solution to fully cover the cubes.
- Incubate for 12 h under gentle shaking at 4 °C.
- Remove potassium permanganate solution and wash the sample several times for 10 min with distilled water until the supernatant remains clear.
- Transfer a piece of the cell mat (corresponding to a volume of approximately 100 µl) into an Eppendorf tube using a 1 ml pipette.
- Dehydration and embedding in Araldite
Note: All incubation steps were performed using a rotator with a rotation speed of 3 rpm.- Dehydrate cells by adding 30%, 50%, 70% and 90% (v/v) ethanol for 15 min and 3x 100% (v/v) ethanol for 20 min. Remove ethanol after each incubation step.
- Incubate the sample for 5 min in propylene oxide (toxic!), remove supernatant and repeat washing step with propylene oxide for 5 min to remove remaining ethanol. Remove supernatant.
- Add freshly prepared Araldite until the cubes are fully covered and incubate for 1 h.
- Remove Araldite and replace by a fresh solution of Araldite. Incubate over night.
- Transfer samples to an embedding mould which is filled with fresh Araldite solution and remove all air bubbles with tweezers.
- Polymerize samples by placing the Araldite filled embedding moulds for 48 h at 60 °C. After polymerization samples can be stored indefinitely.
- Dehydrate cells by adding 30%, 50%, 70% and 90% (v/v) ethanol for 15 min and 3x 100% (v/v) ethanol for 20 min. Remove ethanol after each incubation step.
- Sectioning and post-staining
Note: Sectioning requires a lot of experience. Contacting an expert is recommended for beginners.- Trim blocks with a razor blade and cut sections of 1 µm thickness with an ultramicrotome.
- Transfer a section to a drop of distilled water on a glass slide by using an eyelash attached to a matchstick or a metal loop. Dry section on a hotplate (circa 60 °C).
- Stain sections with 1% (w/v) toluidine blue for 30 sec on hotplate.
- Drain excess stain and rinse section briefly with distilled water.
- Dry on a hotplate and localize cells in a light microscope.
- Reduce size of block further to get the smallest possible block area including the sample of interest and prepare ultrathin sections of approximately 70 nm thickness.
- Transfer the sections to a copper grid.
- Place droplets of saturated aqueous uranyl acetate (toxic!) onto a piece of dental wax in a Petri dish and float the grids (sections downwards) on the droplets for 4 min (Video 1).Video 1.
- Wash the grids by dipping approximately 10 times into distilled water. Repeat three times. (Video 1)
- Blot off excess water with the edge of a piece of filter paper, and transfer the grids to droplets of Reynold's lead citrate placed on dental wax. Stain for 4 min (Video 1).
- Wash with distilled water as described in step 9.
- Blot off excess water and leave the sections to dry before viewing at the TEM. Grids can be used for TEM at any time. For long-term storage (up to 1 year) grids are kept in grid storage boxes (e.g. SPI Slide-A-GridTM storage box). A sample micrograph is shown in Figure 2.
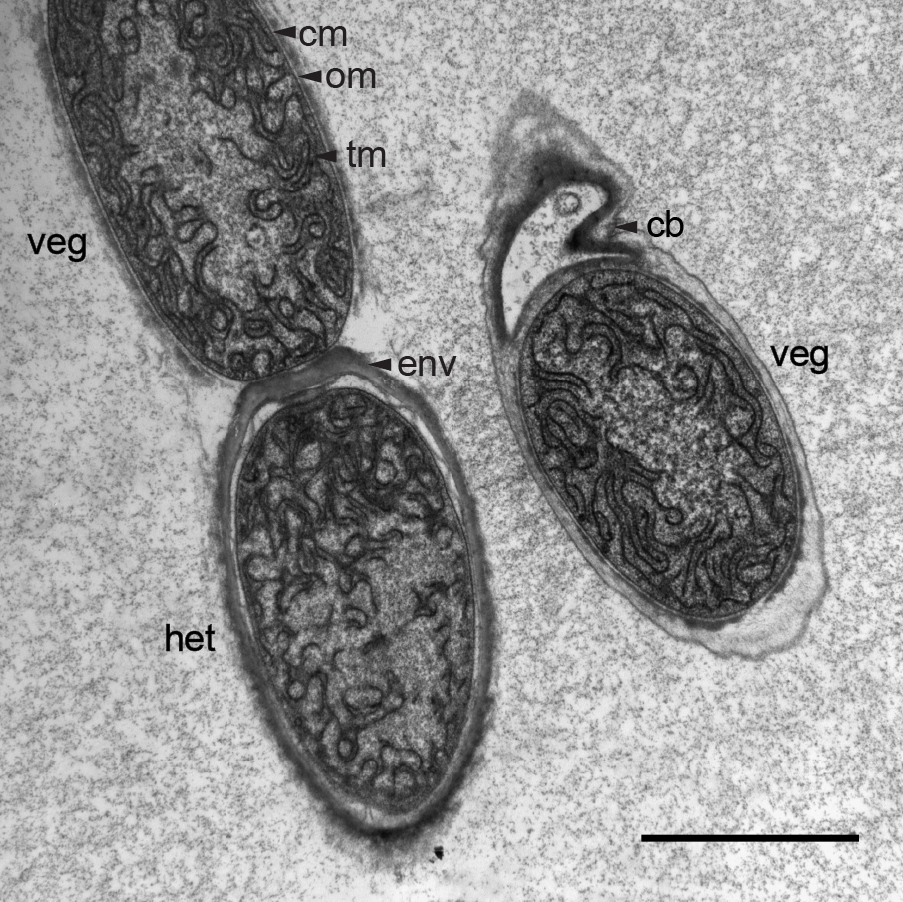
Figure 2. Electron micrograph of an ultra-thin section through a trichome of M. laminosus grown in Castenholz ND medium. Under nitrogen deprivation M. laminosus differentiates photosynthetically active vegetative cells (veg) into nitrogen-fixing heterocysts (het) which possess an additional multilayered envelope (env) as gas diffusion barrier to reduce the entrance of O2 into heterocysts. When filaments break by the formation of necridia ("releasing cells") a small fraction of the former cell remains attached to the trichome (cb). Different membranes are highlighted by black arrows: thylakoid membrane (tm), cytoplasmic membrane (cm) and outer membrane (om). Scale bar, 2 µm
- Trim blocks with a razor blade and cut sections of 1 µm thickness with an ultramicrotome.
Recipes
- 0.125 M Sørensen's phosphate buffer (PB) (pH 7.2-7.4)
450 ml 0.2 M sodium phosphate dibasic (28.4 g in 1,000 ml distilled water)
150 ml 0.2 M potassium phosphate monobasic (27.2 g in 1,000 ml distilled water)
381 ml distilled water
Stored at 4 °C - 4% (v/v) glutaraldehyde in PB
4 ml 25% glutaraldehyde
21 ml PB
Stored at 4 °C
Use within 1 month - 2% (w/v) low gelling temperature agarose
Add 0.2 g agarose to 10 ml PB
Place in the oven at 80 °C and stir occasionally until the agarose is fully dissolved
Stored at 4 °C - 2% (w/v) potassium permanganate
Add 2 g potassium permanganate to 10 ml distilled water
Dissolve by vortexing and filter
Prepare freshly before usage - Araldite
40 ml Araldite CY212
60 ml dodecenylsuccinic anhydride
2 ml methyl nadic anhydride
1 ml benzyl dimethylamine
Stir with a spatula for at least 5 min in a disposable polyethylene beaker; introduce as little air as possible
Leave for 1 h at room temperature
Prepare freshly before usage - 1% (w/v) toluidine blue
Add 1 g toluidine blue to 100 ml 1% (w/v) sodium tetraborate in distilled water
Dissolve by boiling and stir occasionally
Filter and stored at room temperature - Uranyl acetate
Add 1 g uranyl acetate to 100 ml distilled water
Shake vigorously and leave for 48 h to dissolve at room temperature
If all the uranyl acetate has dissolved, add a further 1 g and shake vigorously
Leave for a further 48 h at room temperature
Repeat until no more uranyl acetate dissolves and some undissolved reagent remains - Reynold's lead citrate stain
Dissolve 2.66 g lead nitrate in 30 ml distilled water
Dissolve 3.52 g tri-sodium citrate in 30 ml distilled water
Combine solutions and shake vigorously for 30 sec every 5 min over a period of 30 min
Add 16 ml 1 M sodium hydroxide solution
Mix until solution is clear
Add distilled water to a final volume of 100 ml
Stored at 4 °C
Acknowledgments
This work was supported by a college studentship of Queen Mary University of London.
References
- Castenholz, R. W. (1988). [3] Culturing methods for cyanobacteria. Methods in Enzymology 167: 68-93.
- Luft, J. H. (1956). Permanganate; a new fixative for electron microscopy. J Biophys Biochem Cytol 2(6): 799-802.
- Nurnberg, D. J., Mariscal, V., Parker, J., Mastroianni, G., Flores, E. and Mullineaux, C. W. (2014). Branching and intercellular communication in the Section V cyanobacterium Mastigocladus laminosus, a complex multicellular prokaryote. Mol Microbiol 91(5): 935-949.
Article Information
Copyright
© 2014 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Nürnberg, D. J., Mastroianni, G., Mullineaux, C. W. and McPhail, G. D. (2014). Visualization of Cell Complexity in the Filamentous Cyanobacterium Mastigocladus laminosus by Transmission Electron Microscopy (TEM). Bio-protocol 4(23): e1305. DOI: 10.21769/BioProtoc.1305.
Category
Microbiology > Microbial cell biology > Cell imaging
Cell Biology > Cell imaging > Electron microscopy
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











