- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Elevated Plus Maze Test to Assess Anxiety-like Behavior in the Mouse
Published: Vol 4, Iss 16, Aug 20, 2014 DOI: 10.21769/BioProtoc.1211 Views: 30241
Reviewed by: Soyun KimAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Protocol for Measuring Free (Low-stress) Exploration in Rats
Wojciech Pisula and Klaudia Modlinska
Jan 20, 2020 4459 Views

Operant Vapor Self-administration in Mice
Renata C. N. Marchette [...] Khaled Moussawi
May 20, 2021 4569 Views

Construction of Activity-based Anorexia Mouse Models
Maria Consolata Miletta and Tamas L. Horvath
Aug 5, 2023 1772 Views
Abstract
The elevated plus maze task is a simple method to assess anxiety-like behaviors in rodents. This version describes the procedure used in mice. However, the protocol may also be applied to rats, considering a proportionally larger apparatus (arms: 10 x 50 cm; height: 55 cm). Briefly, the test is performed on a plus-shaped apparatus with two open and two closed arms. The animal is allowed to freely explore the maze for 5 min while the duration and frequency of entries into open and closed arms is recorded. The task is based on an approach-avoidance conflict, meaning that the animal is faced with a struggle between a propensity to explore a novel environment and an unconditioned fear of high and open spaces. Consequently, an anxiety-like state is characterized by increased open arm avoidance, compared to control animals. On account of being a very popular test, there can be considerable variations in the procedures applied across different laboratories. Here we provide a working protocol that has been able to detect both anxiogenic and anxiolyitic drug effects under the specified conditions. Protocol originally published in (Leo et al., 2014).
Materials and Reagents
- Laboratory-bred plus maze-naïve mice
Note: Mice housed in groups of 4-5 per cage, kept in an environment with controlled temperature (around 23 °C) and humidity under a 12-12 h light-dark cycle with food and water ad libitum.
- Paper towels and 70% ethanol for cleaning
Equipment
- Elevated Plus maze (either customized apparatus or commercially available one)
- Video camera (placed directly above the maze)
- Indirect white light
- Digital lux meter (Instrutherm, Mastech, Dr. Meter or similar)
- Digital chronometers for manual analysis, or computer software (Any-maze, Ethovision or others) for automated analysis
Note: In our laboratory, we use a custom made plus-shaped maze sized for mice (Leo et al., 2014), which is elevated 50 cm above the ground, and consists of two opposite closed arms, two opposite open arms and a central square of 5 cm sides (see image below). The closed arms measure 30 x 5 cm and are constituted of white-painted wood, as are the 15 cm high walls that enclose the arms. Glossy painting should be avoided as to prevent excessive glare, thus a matte finish is recommended. The open arms measure 30 x 5 cm and are made of transparent acrylic; a 0.3 cm high transparent acrylic railing prevents animals from falling while exploring the open arms. The apparatus can be disassembled and moved to and from the experiment room where it is set up for use. Wood-made apparatuses covered with wood lacquer, painted in black or transparent Plexiglas apparatuses are also frequently found. Commercially available apparatuses, such as those from Stoelting Co. or Panlab S.L.U., are recommended as alternatives. The room illumination and apparatus light reflection strongly affect the elevated plus maze test. In our laboratory, a Samsung Flashcam digital camcorder is used, as it is more appropriate for posterior off-line analysis. One should always run pilot experiments to establish reliable baseline parameters and standardize the experimental environment before starting the experimental manipulations (drugs, procedure, transgenic animals, etc.).
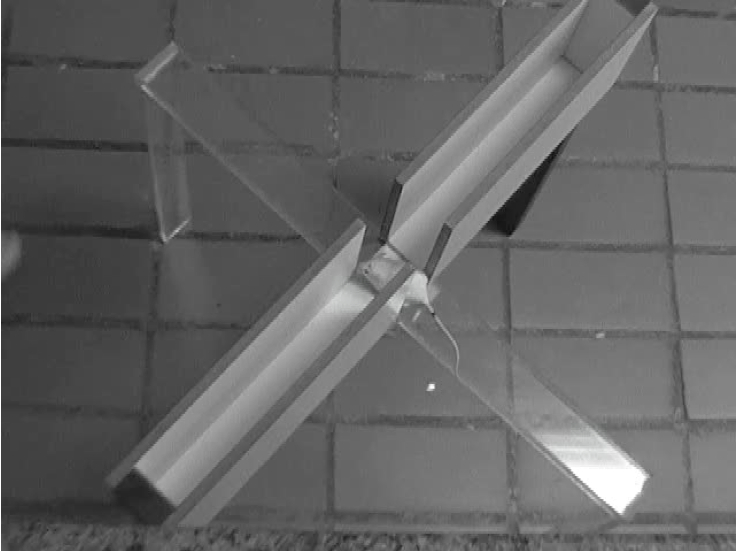
Figure 1. Plus-shaped maze
Software
- Automated computer software (Any-maze, Ethovision and others)
Procedure
- The maze should be assembled in an isolated room away from any extraneous interference of noises, scents or movement. You may choose to place a source of low-intensity white noise in the behavioral experiment room. It should be noted that guidance cues, such as drawings on the walls around the room might skew the animal’s activity to a certain area of the maze. Therefore, any sort of paintings or artifacts that may serve as cues must be removed.
- The experimenter must restraint from making any excessive noise or movement during the entire trial and from wearing perfumes, colognes or any product with a strong smell, since it could act as anxiogenic stimulus for mice.
- Illumination in the room must be measured with the aid of a lux meter, kept constant and controlled according to the analysis that is to be performed. Given that low-intensity luminosity reduces open arm avoidance (Morato and Castrechini, 1989; Bertoglio and Carobrez, 2002; Cosquer et al., 2005; Pereira et al., 2005), to analyze an anxiogenic effect low-intensity lighting (5-30 Lux) should be preferred, whereas an anxiolytic effect should be analyzed under higher intensity lighting (200-400 Lux or more).
- After these experimental conditions are adjusted to a standard, the animals will be brought into the experiment room, where they will be left in their home cages for 45 to 60 min in order to recover from the stress of being moved.
- Clean the maze with 70% ethanol before starting the test in order to remove any dirt or smells accumulated on the apparatus.
- Turn on the video camera and place the first mouse in the center square of the maze facing one of the open arms, preferably the one opposite to the experimenter.
- The experimenter will stand as far away as possible from the maze and out of sight of the test animal, outside of the room if necessary. He must also avoid making unnecessary movement or sounds.
- After 5 min of free exploration, the mouse may be moved out of the maze and back into its home cage.
- All the urine and fecal boli must be removed and the maze cleaned entirely with 70% ethanol to remove any residual smell from the first mouse. Afterwards, the next mouse may be submitted to the test.
- Repeat steps 6-9 until all the animals have been tested.
- The recorded videos can be analyzed by automated computer software or manually with the aid of a chronometer. Several parameters can be considered:
- Entries in closed arms and time spent in closed arms: Measures of total locomotion on the maze throughout the experiment (Carola et al., 2002; Walf and Frye, 2007).
- Entries in open arms and time spent in open arms: May be used as inverse measures of anxiety, that is to say, reduced open arm avoidance reflects lower levels of anxiety (Carola et al., 2002; Walf and Frye, 2007; Griebel et al., 1993).
- Risk-assessment behavior: the frequency and the duration of head-dipping (downward movement of the head towards the floor while on the open arms) and stretch-attend posture (stretched posture with the head and two or three paws on the open arm and retraction to previous position) can be a direct measure of anxiety, i.e. increased risk-assessment behavior indicates higher levels of anxiety (Carola et al., 2002; Weiss et al., 1998; Carobrez and Bertoglio, 2005).
- Entries in closed arms and time spent in closed arms: Measures of total locomotion on the maze throughout the experiment (Carola et al., 2002; Walf and Frye, 2007).
- Statistical analysis: Parameters should be examined separately by analysis of variance across all treatment schedules. The data can be further analyzed by a post-hoc test, if applicable.
Representative data
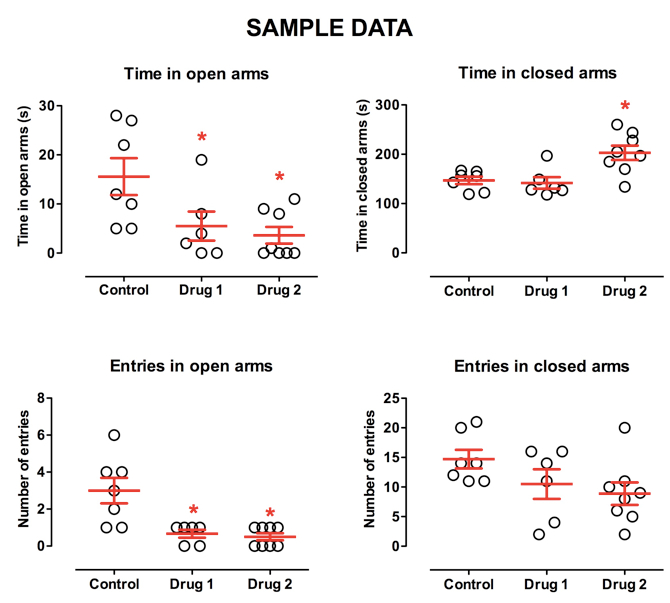
The figure above represents typical data from the elevated plus maze (EPM) in our settings, adapted to investigate anxiogenic responses (10 Lux). The behavior of the control group is relatively distributed, but decent statistically valid data has been gathered with a sample of 6-8 mice per group. Drug 1 is a clear anxiogenic drug, displaying decreased open arm time and open arm entries, without alterations in the time spent and number of entries in the closed arms. Drug 2 suggests a relatively flawed interpretation of an anxiogenic-like pharmacological profile, as treated-mice displayed decreased locomotion, as revealed by the number of entries in each arm. In such case, we recommend a slight reduction in drug dose in order to identify a dose allowing distinction between emotional and locomotor effects induced by the drug. A representative video from one of our experiments published in (Leo et al., 2014) is also available.
Notes
- Typically, male mice are used when performing behavioral experiments, as variations of estrous cycle often influence performance of females in such tests (Simpson et al., 2012; da Silva et al., 2014).
- Baseline activity may vary according to mouse strain, gender and age (Leo et al., 2014; Carobrez and Bertoglio, 2005; Hogg, 1996).
- Animals should be allowed at least one week for habituation to the home cage, as they tend to present high anxiety levels upon arrival from the animal breeder facility.
- In our experiments, animals are only handled for cage cleaning, weighing, drug treatment and during the testing. Also, animals are only tested during the light cycle; testing in the dark cycle may produce different results (Bertoglio and Carobrez, 2002).
- In order to ensure uniform luminosity, an indirect light should always be used to avoid the production of hard shadows, which can be a place of preference for the mouse, skewing its locomotion activity to that area of the maze. This may be done with an appropriate light reflector.
- The maze should always be cleaned after the testing of each animal.
- Make sure the maze is fully dried after cleaning, or a strong smell of the cleaning agent may alter mouse behavior.
- In the event that the mouse falls off the open arm during the test, the experimenter must immediately pick it up and place it back on the open arm. This should be noted and taken into consideration when performing the experiment analysis. This animal can be excluded from the final analysis.
- An entry in the open or closed arm is only considered when the animal places all four paws out of the center square and onto one of the arms. However, two paws out of a given arm, is already sufficient to stop counting the time spent into the arm. If the animal return with these two paws into the arm, the chronometer should be continued, but no entry is registered. A new entry is only registered after a full exit occurs.
- There is no evidence of age-related restrictions for behavioral testing in the elevated plus maze. Despite data supporting increased anxiety-like state in adolescent and aged animals (Lynn and Brown, 2010; Bessa et al., 2005; Chen et al., 2007), the task has been validated for successful assessment of anxiety-like behavior in adolescent, adult, middle-aged and aged rodents (Leo et al., 2014; Warneke et al., 2014; Carrasco et al., 2013; Joshi and Pratico, 2011; Pietrelli et al., 2012; Doremus et al., 2006).
Acknowledgments
The protocol described here has been adapted from a previous study (Leo et al., 2014), which succeeded in identifying anxiogenic-like as well as anxiolytic-like behavior for mice. The project received financial support from FAPERJ-Brasil (E-26/112.448/2012) and CNPq-Brasil (474443/2012-4).
References
- Bertoglio, L. J. and Carobrez, A. P. (2002). Behavioral profile of rats submitted to session 1-session 2 in the elevated plus-maze during diurnal/nocturnal phases and under different illumination conditions. Behav Brain Res 132(2): 135-143.
- Bessa, J. M., Oliveira, M., Cerqueira, J. J., Almeida, O. F. and Sousa, N. (2005). Age-related qualitative shift in emotional behaviour: paradoxical findings after re-exposure of rats in the elevated-plus maze. Behav Brain Res 162(1): 135-142.
- Carobrez, A. P. and Bertoglio, L. J. (2005). Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci Biobehav Rev 29(8): 1193-1205.
- Carola, V., D'Olimpio, F., Brunamonti, E., Mangia, F. and Renzi, P. (2002). Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav Brain Res 134(1-2): 49-57.
- Carrasco, M. C., Vidal, J. and Redolat, R. (2013). Bupropion induced changes in exploratory and anxiety-like behaviour in NMRI male mice depends on the age. Behav Processes 98: 117-124.
- Chen, G. H., Wang, C., Yangcheng, H. Y., Liu, R. Y. and Zhou, J. N. (2007). Age-related changes in anxiety are task-specific in the senescence-accelerated prone mouse 8. Physiol Behav 91(5): 6.
- Cosquer, B., Galani, R., Kuster, N. and Cassel, J.-C. (2005). Whole-body exposure to 2.45 GHz electromagnetic fields does not alter anxiety responses in rats: a plus-maze study including test validation. Behav Brain Res 156(1): 65-74.
- da Silva, C. C., Lazzaretti, C., Fontanive, T., Dartora, D. R., Bauereis, B. and Gamaro, G. D. (2014). Estrogen-dependent effects on behavior, lipid-profile, and glycemic index of ovariectomized rats subjected to chronic restraint stress. Behav Processes 103: 327-333.
- Doremus, T. L., Varlinskaya, E. I. and Spear, L. P. (2006). Factor analysis of elevated plus-maze behavior in adolescent and adult rats. Pharmacol Biochem Behav 83(4): 570-577.
- Griebel, G., Moreau, J.-L., Jenck, F., Martin, J. R. and Misslin, R. (1993). Some critical determinants of the behaviour of rats in the elevated plus-maze. Behav Processes 29(1): 37-47.
- Hogg, S. (1996). A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav 54(1): 21-30.
- Joshi, Y. B. and Pratico, D. (2011). Knockout of 5-lipoxygenase results in age-dependent anxiety-like behavior in female mice. PLoS One 6(12): e29448.
- Leo, L. M., Almeida-Correa, S., Canetti, C. A., Amaral, O. B., Bozza, F. A. and Pamplona, F. A. (2014). Age-dependent relevance of endogenous 5-lipoxygenase derivatives in anxiety-like behavior in mice. PLoS One 9(1): e85009.
- Lynn, D. A. and Brown, G. R. (2010). The ontogeny of anxiety-like behavior in rats from adolescence to adulthood. Dev Psychobiol 52(8): 731-739.
- Morato, S. and Castrechini, P. (1989). Effects of floor surface and environmental illumination on exploratory activity in the elevated plus-maze. Braz J Med Biol Res 22(6): 707-710.
- Pereira, L. O., da Cunha, I. C., Neto, J. M., Paschoalini, M. A. and Faria, M. S. (2005). The gradient of luminosity between open/enclosed arms, and not the absolute level of Lux, predicts the behaviour of rats in the plus maze. Behav Brain Res 159(1): 55-61.
- Pietrelli, A., Lopez-Costa, J., Goni, R., Brusco, A. and Basso, N. (2012). Aerobic exercise prevents age-dependent cognitive decline and reduces anxiety-related behaviors in middle-aged and old rats. Neuroscience 202: 252-266.
- Simpson, J., Ryan, C., Curley, A., Mulcaire, J. and Kelly, J. P. (2012). Sex differences in baseline and drug-induced behavioural responses in classical behavioural tests. Prog Neuropsychopharmacol Biol Psychiatry 37(2): 227-236.
- Walf, A. A. and Frye, C. A. (2007). The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protocols 2(2): 322-328.
- Weiss, S. M., Wadsworth, G., Fletcher, A. and Dourish, C. T. (1998). Utility of ethological analysis to overcome locomotor confounds in elevated maze models of anxiety. Neurosci Biobehav Rev 23(2): 265-271.
- Warneke, W., Klaus, S., Fink, H., Langley-Evans, S. C. and Voigt, J. P. (2014). The impact of cafeteria diet feeding on physiology and anxiety-related behaviour in male and female Sprague-Dawley rats of different ages. Pharmacol Biochem Behav 116: 45-54.
Article Information
Copyright
© 2014 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Leo, L. M. and Pamplona, F. A. (2014). Elevated Plus Maze Test to Assess Anxiety-like Behavior in the Mouse. Bio-protocol 4(16): e1211. DOI: 10.21769/BioProtoc.1211.
Category
Neuroscience > Behavioral neuroscience > Animal model > Mouse
Neuroscience > Behavioral neuroscience > Learning and memory
Neuroscience > Nervous system disorders > Animal model
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








