- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
TRIPLE (Insulin, Glucagon and EGFP) Immunofluorescence Staining Protocol in Pancreas
Published: Vol 4, Iss 5, Mar 5, 2014 DOI: 10.21769/BioProtoc.1056 Views: 15844
Reviewed by: Lin FangFanglian He

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Measuring the Endocytic Recycling of Amyloid Precursor Protein (APP) in Neuro2a Cells
Florent Ubelmann [...] Claudia Guimas Almeida
Dec 5, 2017 8138 Views

Non-separate Mouse Sclerochoroid/RPE/Retina Staining and Whole Mount for the Integral Observation of Subretinal Layer
Sung-Jin Lee and Soo-Young Kim
Jan 5, 2021 4457 Views

Time-Lapse Into Immunofluorescence Imaging Using a Gridded Dish
Nick Lang [...] Andrew D. Stephens
Feb 20, 2026 53 Views
Abstract
This protocol aims to introduce methods for immunostaining two endogenous proteins insulin and glucagon and one exogenous transgene driven EGFP in mouse pancreatic islet. The immunostaining results of insulin and glucagon indirectly tell functionality of pancreatic beta cells and alpha cells respectively. Furthermore, the protocol provides immunostaining steps for the third protein which can be applicable to any other endogenous proteins with a specific antibody generated in mouse.
Materials and Reagents
- Mouse pancreas
- Ethanol (100%, 95%, 85%, 70%, 50%)
- 0.85% NaCl
- ddH2O
- 99.5% Xylene (Sigma-Aldrich, catalog number: 534056 )
- 10% Formalin solution (neutral buffered) (Sigma-Aldrich, catalog number: HT501128 )
- Bovine Serum Albumin (BSA) (Sigma-Aldrich, catalog number: A7906 )
- Tween 20 (Sigma-Aldrich, catalog number: P9416 )
- Triton-X 100 (Sigma-Aldrich, catalog number: T8787 )
- Guinea pig anti-insulin antibody (Millipore, catalog number: 4011-01F )
- Rabbit anti-glucagon antibody (Millipore, catalog number: 4030-01F )
- Mouse anti-EGFP antibody (Clonetech, catalog number: 632381 )
- Texas Red conjugated donkey anti-guinea pig antibody (Jackson ImmunoResearch Laboratories, catalog number: 106-075-003 )
- Alexa 350 conjugated goat anti-rabbit antibody (Life Technologies, InvitrogenTM, catalog number: A-21068 )
- FITC conjugated goat anti-mouse antibody (Jackson ImmunoResearch Laboratories, catalog number: 715-095-166 )
- ProLong Gold Antifade Reagent (Life Technologies, InvitrogenTM, catalog number: P36935 )
- 10x PBS (Gibco®, catalog number: 70011-044 )
- Blocking solution (see Recipes)
Equipment
- Glass jar
- Humidified chamber
- Confocal microscope
- Tissue cassette
Procedure
- Formalin Fixation
- Fix overnight pancreas (~5mm x ~5mm) removed from a mouse in a 15 ml conical tube by 10 ml 10% formalin solution during rotating it at 4 °C.
- On next day, transfer the fixed tissue to a tissue cassette and store it in 70% Ethanol solution till making paraffin block (for at least 1 day) at room temperature.
- Make paraffin blocks of fixed tissues.
- Make slide section of paraffin blocks by Microtome.
- Fix overnight pancreas (~5mm x ~5mm) removed from a mouse in a 15 ml conical tube by 10 ml 10% formalin solution during rotating it at 4 °C.
- Immunostaining of slide sections
- Deparaffinization step
- Keep sections for 5 min in 99.5% Xylene solution 2 times.
- Keep sections for 10 min in 99.5% Xylene solution 2 times.
- Keep sections for 3 min in 100% Ethanol with new glass jar.
- Keep sections for 3 min in 95% Ethanol.
- Keep sections for 3 min in 85% Ethanol.
- Keep sections for 3 min in 70% Ethanol.
- Keep sections for 5 min in 50% Ethanol.
- Keep sections for 5 min in 0.85% NaCl with new glass jar.
- Keep sections for 5 min in 1x PBS.
- Keep sections for 5 min in 99.5% Xylene solution 2 times.
- Permeabilization step
- Incubate sections for 30 min in 0.1% Triton-X 100 in PBS with new glass jar at room temperature (RT).
- Incubate sections for 5 min in 1x PBS with new glass jar 3 times.
- Incubate sections for 30 min in 0.1% Triton-X 100 in PBS with new glass jar at room temperature (RT).
- Blocking, Primary and Secondary Antibody (Ab) binding steps
- Aspirate around the tissue until the slide, not the tissue, is dry. Carefully, trace around the tissue with a grease choke.
- Block for 30 min at RT with 2% BSA in PBS with 0.05% Tween 20 in a coplin jar.
- Aspirate blocking buffer and incubate sections in a humidified camber with glucagon primary Ab (1/100 dilution), in 2% BSA in PBS with 0.05% Tween 20 for overnight (o/n) at 4 °C. Use enough antibody solution (~150 μl) to completely submerge the section.
- Aspirate primary antibody and wash sections for 5 min in 2% BSA in PBS with 0.05% Tween 20 2 times in a coplin jar.
- Aspirate washing buffer and incubate sections in a humidified chamber with corresponding secondary Alexa350 conjugated goat anti-rabbit Ab (1/500 dilution) for 1.15 h at RT in dark. Dilute secondary Ab in 2% BSA in PBS with 0.05% Tween 20. Use enough antibody solution (~150 μl) to completely submerge the section.
- Aspirate secondary antibody and wash sections for 5 min in 2% BSA in PBS with 0.05% Tween 20 2 times in a coplin jar. Aspirate around the tissue until the slide, not the tissue, is dry. And incubate sections in a humidified chamber with Insulin primary Ab (1/500 dilution), in 2% BSA in PBS with 0.05% Tween 20 for 1.5 h at RT in dark. Use enough antibody solution (~150 μl) to completely submerge the section.
- Aspirate primary antibody and wash sections for 5 min in 2% BSA in PBS with 0.05% Tween 20 2 times at RT in dark in a coplin jar.
- Aspirate around the tissue until the slide, not the tissue, is dry. And Incubate sections in a humidified chamber with corresponding secondary Texas Red conjugated donkey anti-guinea pig Ab for 1 h at RT in dark. Dilute secondary Ab (1/1,000 dilution) in 2% BSA in PBS with 0.05% Tween 20. Use enough antibody solution (~150 μl) to completely submerge the section.
- Aspirate secondary antibody and wash sections for 5 min in 2% BSA in PBS with 0.05% Tween 20 2 times at RT in dark in a coplin jar. Aspirate around the tissue until the slide, not the tissue, is dry. And incubate sections in a humidified camber with EGFP primary Ab (1/500 dilution), for 1 h at 4 °C in dark. Diluted primary Ab in 2% BSA in PBS with 0.05% Tween 20. Use enough antibody solution (~150 μl) to completely submerge the section.
- Aspirate primary antibody and wash sections for 5 min in 2% BSA in PBS with 0.05% Tween 20 2 times at RT in dark in a coplin jar.
- Aspirate washing buffer and Incubate sections in a humidified chamber with corresponding secondary FITC conjugated goat anti-mouse Ab (1/500 dilution) for 1 h at RT in dark. Dilute secondary Ab in 2% BSA in PBS with 0.05% Tween 20. Use enough antibody solution (~150 μl) to completely submerge the section.
- Aspirate secondary antibody and wash sections for 5 min in 2% BSA in PBS with 0.05% Tween 20 2 times at RT in dark in a coplin jar.
- Aspirate around the tissue until the slide, not the tissue, is dry. Carefully, trace around the tissue with a grease choke.
- Deparaffinization step
- Mounting Step
- Aspirate around the tissue until the slide, not the tissue, is dry, Add ~50 μl of ProLong® Gold Antifade Reagent containing DAPI to each section and place a cover slip over section.
Note: Be careful to avoid damaging the tissue by sliding the cover slip too much and do not introduce bubbles. Aspirate excess reagent. - Dry sections at room temperature for several hours or overnight protected from light.
- Once the slides are completely dry, seal the edges of the cover slip with clear nail polish. Slides can be stored at -20 °C, protected from light for several weeks.
- Aspirate around the tissue until the slide, not the tissue, is dry, Add ~50 μl of ProLong® Gold Antifade Reagent containing DAPI to each section and place a cover slip over section.
Representative data
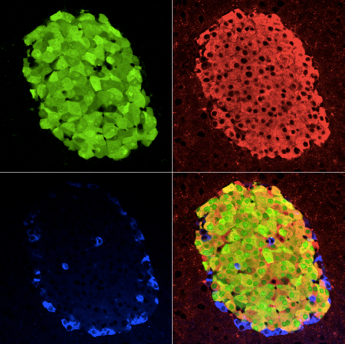
Figure 1. Triple immunostaining for EGFR, insulin, and glucagon in a pancreatic tissue section. Pancreatic tissue sections were obtained from mice 3 weeks after Tam administration to induce EGFP from a transgene. Pancreatic tissue sections were triple immunostained for EGFP (Green), insulin (Red), and glucagon (Blue), and representative single-channel fluorescence images are shown individually and merged (lower-right image of each group). [Please cite Reference 1 (Figure 1B)]
Recipes
- Blocking solution (2% BSA in PBS pH 7.4 with 0.05% Tween20)
10 g BSA
50 ml 10x PBS
0.25 ml Tween20
Add ddH2O to 500 ml
Acknowledgments
This work was supported in part by the National Research Foundation of Korea funded by the Ministry of Education, Science, and Technology Grants 2011-0011433, 2012M3A9C3048686 and 2014R1A1A4A01004329 (S.H.B) and NIH grants DK42394, HL52173, and HL057346 (R.J.K.). R.J.K. was an Investigator of the Howard Hughes Medical Institute. This protocol was adapted from Back et al. (2009), and a first short version of the adapted protocol was published in Han et al. (2013).
References
- Back, S. H.*, Scheuner, D.*, Han, J., Song, B., Ribick, M., Wang, J., Gildersleeve, R. D., Pennathur, S. and Kaufman, R. J. (2009). Translation attenuation through eIF2alpha phosphorylation prevents oxidative stress and maintains the differentiated state in beta cells. Cell Metab 10(1): 13-26. *These authors contributed equally to this work
- Han, J.*, Back, S. H.*, Hur, J., Lin, Y. H., Gildersleeve, R., Shan, J., Yuan, C. L., Krokowski, D., Wang, S., Hatzoglou, M., Kilberg, M. S., Sartor, M. A. and Kaufman, R. J. (2013). ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol 15(5): 481-490. *These authors contributed equally to this work
Article Information
Copyright
© 2014 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Choi, W., Kaufman, R. J. and Back, S. H. (2014). TRIPLE (Insulin, Glucagon and EGFP) Immunofluorescence Staining Protocol in Pancreas. Bio-protocol 4(5): e1056. DOI: 10.21769/BioProtoc.1056.
Category
Cell Biology > Cell imaging > Fluorescence
Biochemistry > Protein > Immunodetection > Immunostaining
Cell Biology > Cell staining > Protein
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link