- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Intestinal Differentiation of Mouse ESCs
Published: Vol 3, Iss 24, Dec 20, 2013 DOI: 10.21769/BioProtoc.1011 Views: 8567
Reviewed by: Salma HasanLin FangAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Differentiation, Maintenance, and Contraction Profiling of Human Induced Pluripotent Stem Cell–Derived Cardiomyocytes
Matthijs Snelders [...] Jeroen Essers
Mar 5, 2025 3937 Views

Isolation and Culture of Ferret Airway Stem Cells
Ziying Yan [...] Feng Yuan
Jul 20, 2025 2413 Views

Optimization of Adipogenic Differentiation Protocol for Murine and Human Cell Culture Models
Junwan Fan [...] Wenyan He
Jan 20, 2026 230 Views
Abstract
ES cells (ESCs) are pluripotent and offer a good tool to study early embryonic development. Intestinal cells are derived from the definitive endoderm. In contrast to adult tissue stem cells, embryonic development and differentiation from ES cells have not been as well investigated in the intestine. There are four differentiated cell types of non-proliferative epithelial cells: enterocytes, goblet cells, enteroendocrine cells, and Paneth cells. Intestinal stem cells (ISCs) and progenitor cells reside in crypts, proliferate vigorously, and function as the source of differentiated epithelial cells. Here, we describe a protocol, in which differentiated cell types of the intestine are derived from mouse ESCs. In this protocol, we describe a protocol to differentiate mouse ES cells into Cdx2-expressing intestinal endoderm efficiently by co-culturing with M15, a mouse mesonephric cell line, and treatment with two chemical compounds. The chemical compounds used are BIO and DAPT. BIO is a Gsk3 inhibitor, that activate Wnt signaling pathway, and DAPT is a-secretase inhibitor that inhibit Notch signaling pathway. BIO and DAPT treatment yielded all representative cell lineages, enterocytes, goblet cells, enteroendocrine cells and paneth cells, to be derived from mouse ESCs.
Materials and Reagents
- Mouse embryonic stem cells (ESCs)
- M15 cells (ECACC: catalog number: 95102517 )
- Mouse embryonic fibroblast (MEF)
- 0.1% gelatin
- PBS
- Dulbecco’s Modified Eagle Medium (DMEM) high glucose (Gibco®, catalog number: 11995-075)
- DMEM low glucose (Gibco®, catalog number: 11885-084 )
- Fetal Bovine Serum (FBS) (Hyclone, catalog number: SH30310.03 )
- 200 mM L-glutamine (L-Gln) (Nacalai Tesque, catalog number: 16948-04 )
- 5,000 units/ml mixture of penicillin and streptomycin (PS) (Nacalai Tesque, catalog number: 26252-94 )
- 10 mM MEM Non-Essential Amino Acids Solution (NEAA) (Gibco®, catalog number: 11140-050 )
- 2-mercaptoethanol (2-ME) (Sigma-Aldrich, catalog number: M7522 )
- D-(+)-Glucose (Sigma-Aldrich, catalog number: G5146 )
- KnockOut Serum Replacement (KSR) (Gibco®, catalog number: 10828 )
- Recombinant human LIF (Wako Pure Chemical Industries, catalog number: 129-05601 )
- Recombinant human Activin A (R&D Systems, catalog number: 338-AC )
- Recombinant human bFGF (Pepro Tech, catalog number: 100-18B )
- BIO (Calbiochem®, catalog number: 361550 )
- DAPT (Wako Pure Chemical Industries, catalog number: 041-30983 )
- Mitomycin C (MMC) (Sigma-Aldrich, catalog number: M4287 )
- 0.05% Trypsin-EDTA (Life Technologies, catalog number: 2014-11)
- 0.25% Trypsin-EDTA (Life Technologies, catalog number: 25200-072 )
- EF medium (see Recipes)
- Maintenance Medium (see Recipes)
- EB medium (see Recipes)
- Endoderm Medium (see Recipes)
- Intestinal Medium (see Recipes)
Equipment
- 6 well plate (Corning, catalog number: 3516)
- 100 mm dish (Corning, catalog number: 430167 )
- 60 mm dish (BD Biosciences, Falcon®, catalog number: 353004 )
- Centrifuge
- 37 °C 5% CO2 Cell culture incubator
Procedure
- Preparation of M15 cells
- M15 cells (5x106) are plated to a 100 mm dish with 10 ml EF medium.
- M15 cells are incubated in the 5% CO2 incubator at 37 °C.
- When M15 cells are confluent (for 2-3 days), passage from a 100 mm dish to five 100 mm dishes.
- M15 cells are washed by PBS.
- M15 cells are trypsinized with 1 ml 0.05% Trypsin-EDTA at 37 °C for 5 min.
- M15 cells are pelleted at 190 x g for 5 min.
- M15 cells are plated to five 100 mm dishes.
- M15 cells are washed by PBS.
- When M15 cells are confluent, M15 cells are incubated with 10 μg/ml MMC containing EF medium in the 5% CO2 incubator at 37 °C for 2 h. M15 cells are treated with MMC to stop M15 cell from proliferation.
- MMC-treated M15 cells are washed by PBS.
- MMC-treated M15 cells are trypsinized with 1 ml 0.05% Trypsin-EDTA at 37 °C for 5 min.
- M15 cells are pelleted at 190 x g for 5 min.
- Resuspend and freeze MMC-treated M15 cells into cryovials in 22% FBS and 20% DMSO containing DMEM at 1 x 107 cells per tube at -150 °C.
- M15 cells (5x106) are plated to a 100 mm dish with 10 ml EF medium.
- Preparation of MEF
- MEF (5x106) are plated to a 100 mm dish with 10 ml EF medium.
- MEF are incubated in the 5% CO2 incubator at 37 °C.
- When MEF are confluent (for 2-3 days), passage from a 100 mm dish to five 100 mm dishes.
- MEF are washed by PBS.
- MEF are trypsinized with 1 ml 0.05% Trypsin-EDTA at 37 °C for 5 min.
- MEF are pelleted at 190 x g for 5 min.
- MEF cells are plated to five 100 mm dishes.
- MEF are washed by PBS.
- When MEF cells are confluent, MEF are incubated with 10 μg/ml MMC containing EF medium in the 5% CO2 incubator at 37 °C for 2 h. MEF are treated with MMC to stop M15 cell from proliferation.
- MMC-treated MEF are washed by PBS.
- MMC-treated MEF are trypsinized with 1 ml 0.05% Trypsin-EDTA at 37 °C for 5 min.
- M15 cells are pelleted at 190 x g for 5 min.
- Resuspend and freeze MMC-treated M15 cells into cryovials in 22% FBS and 20% DMSO containing DMEM at 2 x 106 cells per tube at -150 °C.
- MEF (5x106) are plated to a 100 mm dish with 10 ml EF medium.
- Maintenance of mouse ESCs
- 2 x 106 MMC-treated MEF cells are plated to a gelatin-precoated 60 mm dish with EF medium.
- MMC-treated MEF cells are incubated in the 5% CO2 incubator at 37 °C overnight.
- Mouse ESCs (5x105) are plated on MEF-coated dish with Maintenance Medium, and are incubated in the 5% CO2 incubator at 37 °C (day 0) until passage (3 days).
- Medium is changed every day.
- 2 x 106 MMC-treated MEF cells are plated to a gelatin-precoated 60 mm dish with EF medium.
- Intestinal differentiation of mouse ESCs
- 1 x 107 MMC-treated M15 cells are plated to two gelatin-coated 6 well plates and are incubated in the 5% CO2 incubator at 37 °C overnight.
- ESCs plated on MEF-coated dishes (step B3) are washed by PBS.
- ESCs are trypsinized with 1 ml 0.25% Trypsin-EDTA at 37 °C for 5 min.
- ESC cells are pelleted at 190 x g for 5 min.
- The supernatant is removed and 1 x 106 cells/ml resuspended in the EB Medium.
- 2 x 104 ESCs in 2 ml EB medium are plated to a M15-pre-coated well (day 0).
- Medium is changed to Endoderm Medium at day 1 and 3.
- Medium is changed to the Intestinal Medium at day 5.
- Medium is changed with 2 ml Intestinal Medium at day 7, 9, 11, 13, 15, 17 and 19 (Figure 1).
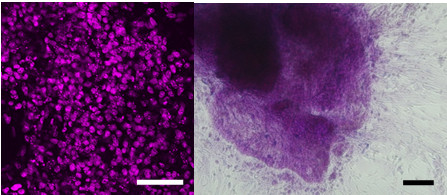
Figure 1. Mouse ESC-derived Cdx2 (red)-expressing intestinal endoderm at day12 (left) and PAS-positive mucus secreting functional goblet cell at day 20 (Right). Scale bar indicates 100 μm.
- 1 x 107 MMC-treated M15 cells are plated to two gelatin-coated 6 well plates and are incubated in the 5% CO2 incubator at 37 °C overnight.
Recipes
- EF medium
DMEM high glucose containing 10% FBS
2 mM L-Gln
50 units/ml PS
- Maintenance Medium
DMEM high glucose containing 10% FBS
2 mM L-Gln
50 units/ml PS
100 μM NEAA
100 μM 2-ME
LIF
- EB Medium
DMEM high glucose containing 10% FBS
2 mM L-Gln
50 units/ml PS
100 μM NEAA
100 μM 2-ME
- Endoderm Medium
EB medium with 10 ng/ml Activin A
5 ng/ml bFGF
- Intestinal Medium
2 ml DMEM low glucose with 1 mg/ml D-(+)-Glucose
10% KSR
2 mM L-Gln
50 units/ml PS
100 μM NEAA
100 μM 2-ME
5 μM BIO
10 μM DAPT
Acknowledgments
This protocol is adapted from Ogaki et al. (2013). This work was supported by a Grant-in-Aid (#21390280 to S.K. and #21790671 to N.S.), and in part by a Global COE grant (Cell Fate Regulation Research and Education Unit, to S.K.), and a grant from the Project for Realization of Regenerative Medicine from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) Japan.
References
- Ogaki, S., Shiraki, N., Kume, K. and Kume, S. (2013). Wnt and Notch signals guide embryonic stem cell differentiation into the intestinal lineages. Stem Cells 31(6): 1086-1096.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Ogaki, S. and Kume, S. (2013). Intestinal Differentiation of Mouse ESCs. Bio-protocol 3(24): e1011. DOI: 10.21769/BioProtoc.1011.
Category
Stem Cell > Embryonic stem cell > Maintenance and differentiation
Cell Biology > Cell isolation and culture > Cell differentiation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link