- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Evaluation of Caspase-1 Activation and IL-1β Production in A Kainic Acid Microdyalisis Brain Injury Model
Published: Vol 3, Iss 8, Apr 20, 2013 DOI: 10.21769/BioProtoc.433 Views: 10666

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Gas Chromatography Detection Protocol of Short-chain Fatty Acids in Mice Feces
Chaozheng Zhang [...] Hua Zhao
Jul 5, 2020 6983 Views

Maternal Immune Activation with the Viral Mimetic Poly:IC in Pregnant Rats
Thaisa Meira Sandini [...] John George Howland
Nov 20, 2020 4179 Views

Using the Cecal Ligation and Puncture Model of Sepsis to Induce Rats to Multiple Organ Dysfunction
Jose Manuel Condor Capcha [...] Samirah A. Gomes
Apr 5, 2021 5825 Views
Abstract
Intracerebral infusion of kainic acid (KA) by a microdialysis probe induces a focal swelling in the brain-perfused area which promotes inflammation (Compan et al., 2012; Oprica et al., 2003). The microdialysis technique allows the local in vivo perfusion of KA and the simultaneous collection of inflammatory mediators, and other neuroactive substances, released in the injured brain. This protocol also allows the perfusion of different solutions in each cerebral hemisphere at the same time. By perfusing KA in isotonic solution of Krebs-Ringer Bicarbonate (KRB) (280-290 mOsm) in one hippocampus and KA in hypertonic KRB solution (1,400-1,500 mOsm) in the contralateral side, we can evaluate in vivo the efficiency of hypertonic solutions in preventing inflammation induced by swelling after KA infusion. Once the inflammatory response has been induced, it is possible to infuse through the microdialysis probe a biotinylated specific inhibitor of caspase-1 allowing the detection of the brain regions and cells involved in IL-1 production in response to the injury (Oprica et al., 2003).
Keywords: ELISAMaterials and Reagents
- Female Sprague-Dawley rats* of 200-220 g body weight
- Isoflurane (Baxter S. L, catalog number: 691477H )
- Local analgesic Bupivacaine 0.25% (Braun Medical S. A, catalog number: 671065 )
- Betadine (Meda Farma Sau, catalog number: 644625.6 )
- Ocular lubricant (Lab Pérez Giménez, catalog number: 973370 )
- Sorbitol (Sigma-Aldrich, catalog number: S6021 )
- Albumin from rat serum (Sigma-Aldrich, catalog number: A6414 )
- Kainic Acid 100 mM (Sigma-Aldrich, catalog number: K0250 )
- Biotinylated Caspase Inhibitor 100 mM biotinyl-YVAD-CMK (AnaSpec, USA)
- Capillary Gap microscope slides (Dako REAL, catalog number: K8020 )
- Fluorescein-streptavidin conjugate (BD Pharmingen, PharmingenTM, catalog number: 554060 A-2001 )
- NeuN (Chemicon International, catalog number: MAB377 )
- Goat anti-mouse IgG secondary antibody Alexa Fluor-568 (Life Technologies, Molecular Probes®, catalog number: A11031 )
- Hoechst 33342 (Sigma-Aldrich, catalog number: B2261 )
- Paraformaldehyde
- KH2PO4
- MgSO4
- NaHCO3
- CaCl2
- KCl
- Isotonic Krebs Ringer Bicarbonate (see Recipes)
- Hypertonic KRB (see Recipes)
- Isotonic Krebs Ringer Bicarbonate (KRB) (see Recipes)
- Hypertonic KRB (see Recipes)
- Sacrifice anesthesia solution (see Recipes)
Equipment
- Small-animal sterotaxic apparatus (David Kopf, catalog number: 962 )
- Perfusion pumps (Bas, catalog number: MD-1001 )
- 1 ml syringes (Bas, catalog number: MD-000 )
- Dental drill and # 1 burrs
- Scalpel
- Anesthesia Isoflurane vaporizer (Parkland Scientific, catalog number: V3000PK )
- Rat ear bars (David Kopf, catalog number: 957 )
- Rat anesthesia mask (David Kopf, catalog number: 1929-B )
- Hypodermic needle (25 G)
- Microdialysis pumps (BAS, catalog number: MD-1001 Babe Bee)
- Microdialysis probes, Cut-off 100.000 Daltons CMA-12 14/02 PES, (CMA, USA)
- Liquid Switch Valve (Univentor, Zejtun ZTN08, catalog number: 8401405 )
- Tygon tubing R3603; 0.38 mm ID (Cole Parmer Instruments Co. USA)
Experimental set-up
Procedure
- Animal Preparation
- The rat must be anesthetized prior to be placed in the stereotaxic equipment instrument. Place the animal into a vaporizer isoflurane box (breathing chamber), and keep it until the rat it is completely anesthetized. The anesthetic system automatically mixes pure isoflurane solution with oxygen in a desired proportion. During the performance of the experiment, a 2% mix of isoflurane is used.
- Shave the hair from the head.
- Stabilize the head of the animal by using rat ear bars and the rat anesthesia mask, adjusted dorso-ventrally at -3.0. To properly mark the coordinates for the microdialysis probes implant, the incisor bar was adjusted until the heights of lambda and bregma were equal. This flat skull position was achieved when the incisor bar was lowered 3.3 ± 0.4 mm below horizontal zero, according to Paxinos atlas (Paxinos and Watson, 1997).
- Apply ocular lubricant on eyes to avoid drying up.
- Decontaminate the skin with Betadine applying it on the head of the rat with the aid of a tip of cotton.
- To avoid extra bleeding from the skull, we administrate a vasoconstrictor local analgesic subcutaneously in the middle line of the head (0.1-0.2 ml).
- Make a 1-1.5 cm incision in the middle line of the skin head.
- Identify bregma and lambda after gently scraping away the periosteal connective tissue.
- The rat must be anesthetized prior to be placed in the stereotaxic equipment instrument. Place the animal into a vaporizer isoflurane box (breathing chamber), and keep it until the rat it is completely anesthetized. The anesthetic system automatically mixes pure isoflurane solution with oxygen in a desired proportion. During the performance of the experiment, a 2% mix of isoflurane is used.
- Microdialysis probes implant
- According to the Paxinos atlas (Paxinos and Watson, 1997) the coordinates used for the implant of microdialysis probes are: - 4.6 mm posterior to bregma, + 2.5 mm lateral to midline (right hemisphere), - 2.5 mm lateral to midline (left hemisphere) and + 3.0 mm ventral from dura mater.
- Mark stereotaxically these points in order to drill the bone for the insertion of the microdialysis probes. The holes must penetrate the full skull but not the dura mater. The dura mater can be cut with a hypodermic needle (25 G). Apply lint if a small bleeding is produced.
- Mount the microdialysis probe in the probe/guide holder and attach it to a sterotaxic manipulator.
- Connect syringes to the liquid switch valve and then to the inlet canula of the probe through a 500 mm length Tygon tubing.
- Connect 25 mm of Tygon tubing to the outlet canula of the probe to collect the perfusate.
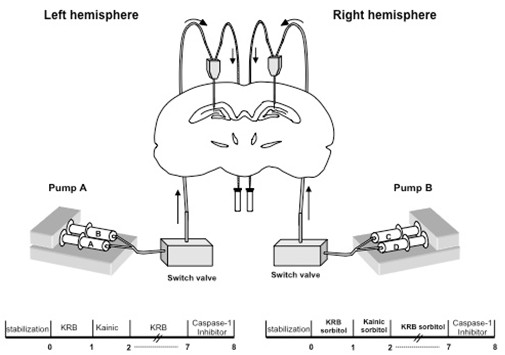
- Implant one probe in each hemisphere.
- In the left hemisphere we have two syringes connected to the probe: Syringe A filled with isotonic KRB and syringe B with isotonic KRB + Kainic Acid. Both connected to a microdialysis pump. Run the pump at flow rate of 1 μl/min.
- In the right hemisphere, we also have two syringes: Syringe C is filled with hypertonic KRB and syringe B with hypertonic KRB + Kainic Acid. Both connected to the microdyalysis pump.
- Start KRB perfusion in the left hemisphere immediately after the probe implant. The first 100 min of perfusion are discarded (stabilization period). After this period, collect the basal sample for 1 h. Then, perfuse kainic acid for 60 min, followed by a washout period of 6 h. At the end of the experiment, perfuse the biotinylated caspase-1 inhibitor for 1 h. The same protocol is used for the right hemisphere perfusion.
- Collect samples in an Eppendorf tube every hour and immediately transfer them to the freezer at -20 °C for its posterior IL-1 analysis.
- Stitch up the skin head and transfer the animal to a cage overnight.
- According to the Paxinos atlas (Paxinos and Watson, 1997) the coordinates used for the implant of microdialysis probes are: - 4.6 mm posterior to bregma, + 2.5 mm lateral to midline (right hemisphere), - 2.5 mm lateral to midline (left hemisphere) and + 3.0 mm ventral from dura mater.
- Tissue processing and histological detection of caspase-1 inhibitor
- Sacrifice the animal the following day under deep sacrifice anesthesia solution through intracardiac perfusion fixation with 250 ml of 4% paraformaldehyde, after a previous perfusion wash with 50 ml isotonic saline.
- Postfix brain in the same fixative solution for 24 h at 4 °C, wash and cryoprotect the tissue with 30% sucrose in phosphate-buffered saline (PBS). Freeze down the tissue and cut 20-μm-thick coronal sections of the hyppocampus in a cryostat.
- Mount tissue sections on positive-charged slides (Capillary Gap microscope slides), and treat them with 10 mM sodium acetate (pH 6.0) at 95 °C for 4 min for antigen retrieval.
- Incubate sections in fluorescein-streptavidin conjugate (1:200) for fluorochrome detection of caspase-1 inhibitor.
- For double-staining with a neuronal marker, incubate sections with the anti-neuronal nuclei primary antibody NeuN (1:500), after a previous treatment with 5% normal goat serum in 0.1% Triton X-100 in PBS.
- Incubate sections with Alexa Fluor-568 goat anti-mouse IgG secondary antibody (1:400).
- Coverslip slides in a medium containing p-phenylenediamine and bisbenzimide (Hoechst 33342) for nuclei detection.
Note: All procedures used in this work were in accordance with the European Union Council Directive (86/609/EEC). The protocol was approved by the Committee on the Ethics of Animal Experiments of the Hospital “Ramón y Cajal” (animal facilities ES280790002001).
- Sacrifice the animal the following day under deep sacrifice anesthesia solution through intracardiac perfusion fixation with 250 ml of 4% paraformaldehyde, after a previous perfusion wash with 50 ml isotonic saline.
Recipes
- Isotonic Krebs Ringer Bicarbonate (KRB)
0.4 mM KH2PO4
1.2 mM MgSO4
25 mM NaHCO3
1.2 mM CaCl2
3 mM KCl
0.5 mg/ml Rat Albumin - Hypertonic KRB
0.4 mM KH2PO4
1.2 mM MgSO4
25 mM NaHCO3
1.2 mM CaCl2
3 mM KCl
20% Sorbitol
0.5 mg/ml Rat Albumin - Sacrifice anesthesia solution
0.4-0.5 ml/rat of a mixture prepared as follows:
0.5 ml of Ketamine (50 mg/ml)
0.4 ml of Valium (4 mg/ml)
0.1 ml Atropine (1 mg/ml)
Acknowledgments
This protocol has been adapted from the previously published paper: Compan et al. (2012). This work was supported by grants from PN I+D+I 2008-2011-Instituto Salud Carlos III-FEDER (EMER07/049 and PI09/0120) and Fundación Séneca (11922/PI/09). The authors declare no conflict of interests.
References
- Compan, V., Baroja-Mazo, A., López-Castejón, G., Gomez, A. I., Martínez, C. M., Angosto, D., Montero, M. T., Herranz, A. S., Bazán, E. and Reimers, D. (2012). Cell volume regulation modulates NLRP3 inflammasome activation. Immunity 2012;37:487-500.
- Oprica, M., Eriksson, C. and Schultzberg, M. (2003). Inflammatory mechanisms associated with brain damage induced by kainic acid with special reference to the interleukin‐1 system. J Cell Mol Med 7(2): 127-140.
- Paxinos G and Watson C. The rat brain in sterotaxic coordinates, 3ed Edition.(1997). Academic Press, Inc. (Ca, USA)
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Herranz, A. S., Bazán, E., Reimers, D., Montero-Vega, M. T., Jménez-Escrig, A. and Pelegrín, P. (2013). Evaluation of Caspase-1 Activation and IL-1β Production in A Kainic Acid Microdyalisis Brain Injury Model. Bio-protocol 3(8): e433. DOI: 10.21769/BioProtoc.433.
Category
Immunology > Animal model > Rat
Biochemistry > Other compound > Acid
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link