- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Quantification of 2-Hydroxyglutarate Enantiomers by Liquid Chromatography-mass Spectrometry
Published: Vol 6, Iss 16, Aug 20, 2016 DOI: 10.21769/BioProtoc.1908 Views: 11868
Reviewed by: Masahiro MoritaShannon RuppertPamela Maher

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Measurement of 2-methylthio Modifications in Mitochondrial Transfer RNAs by Reverse-transcription Quantitative PCR
Fan-Yan Wei and Kazuhito Tomizawa
Jan 5, 2016 9528 Views

Analysis of Leukemia Cell Metabolism through Stable Isotope Tracing in Mice
Nick van Gastel [...] David T. Scadden
Oct 5, 2021 5229 Views
Abstract
Two enantiomers of 2-hydroxyglutarate (2HG), L (L2HG) and D (D2HG), are metabolites of unknown function in mammalian cells that were initially associated with separate and rare inborn errors of metabolism resulting in increased urinary excretion of 2HG linked to neurological deficits in children (Chalmers et al., 1980; Duran et al., 1980; Kranendijk et al., 2012). More recently, investigators have shown that D2HG is produced by mutant isocitrate dehydrogenase enzymes associated with a variety of human malignancies, such as acute myeloid leukemia, glioblastoma multiforme, and cholangiocarcinoma (Cairns and Mak, 2013; Dang et al., 2009; Ward et al., 2010). By contrast, we and others have shown that L2HG accumulates in response to cellular reductive stressors like hypoxia, activation of hypoxia inducible factors, and mitochondrial electron transport chain defects (Oldham et al., 2015; Reinecke et al., 2011; Intlekofer et al., 2015; Mullen et al., 2015). Each enantiomer is produced and metabolized in independent biochemical pathways in reactions catalyzed by separate enzymes and utilizing different cofactors with presumably different consequences for cellular metabolism (Kranendijk et al., 2012). Therefore, as research into the roles of D2HG and L2HG in human metabolism continues, it becomes increasingly important for investigators to consider each enantiomer independently (Struys, 2013). Several methods for quantification of biochemically relevant enantiomers in general have been developed and typically include enzymatic assays using enzymes specific for one enantiomeric species or the other, the use of chiral chromatography medium to facilitate chromatographic separation of enantiomers prior to spectroscopy, or the use of chiral derivatization reagents to convert a mixture of enantiomers to diastereomers with differing physical and chemical properties facilitating their chromatographic separation. In this protocol, we report the adaptation of a previously published derivatization method using diacetyl-L-tartaric anhydride (DATAN) for the quantification of 2HG enantiomers (Figure 1) (Oldham et al., 2015; Struys et al., 2004).
Figure 1. Reaction scheme for the derivatization protocol
Materials and Reagents
- Screw cap 2 ml microcentrifuge tubes (Fisher Scientific, catalog number: 21-403-200 )
- D-2-hydroxyglutaric acid disodium salt (Sigma-Aldrich, catalog number: H8378 )
- L-2-hydroxyglutaric acid disodium salt (Sigma-Aldrich, catalog number: 90790 )
- Sodium lactate (Sigma-Aldrich, catalog number: L7022 )
- Water (LC-MS grade) (Thermo Fisher Scientific, catalog number: W6 )
- [13C4]-2-oxoglutaric acid disodium salt (Cambridge Isotope Labs, catalog number: CLM-4442 )
- Methanol, Optima (LC-MS grade) (Thermo Fisher Scientific, catalog number: A454 )
- Diacetyl-L-tartaric anhydride (DATAN) (Sigma-Aldrich, catalog number: 358924 )
- Acetonitrile, Optima (LC-MS grade) (Fisher Scientific, catalog number: A955 )
- Acetic Acid, Glacial (Fisher Scientific, catalog number: BP2401 )
- Formic Acid, Optima (LC-MS grade) (Fisher Scientific, catalog number: A117 )
- Ammonium Hydroxide (Fisher Scientific, catalog number: A669 )
- Biological samples (see Note 1)
- 50 mM D2HG and L2HG stock solution (see Recipes)
- 20 μM 2HG working solution (see Recipes)
- 10 mM sodium lactate stock solution (see Recipes)
- 500 μM internal standard (ISTD) stock solution (see Recipes)
- Buffer A (see Recipes)
- Buffer B (see Recipes)
Equipment
- Savant SpeedVac ISS110
Note: This device has been discontinued by Thermo Fisher Scientific. - Isotemp digital dry bath incubator (Fisher Scientific, catalog number: 11-715-125DQ )
- Sequant ZIC-HILIC HPLC column (3.5 μm, 100 Å, 2.1 mm internal diameter, 150 mm length) (Merck Millipore Corporation, catalog number: 1504420001 )
- Sequant ZIC-HILIC HPLC guard column (Merck Millipore Corporation, catalog number: 1504360001 )
- Surveyor autosampler plus
Note: This device has been discontinued by Thermo Fisher Scientific. - Surveyor MS Pump Plus
Note: This device has been discontinued by Thermo Fisher Scientific. - Finnigan LTQ mass spectrometer
Note: This device has been discontinued by Thermo Fisher Scientific.
Software
- Xcalibur software (Thermo Fisher Scientific)
Procedure
- Preparation of standards
- Standards are prepared in 80% methanol by combining 0-20 μl of the racemic 20 μM 2HG working solution with 2 μl each of the 10 mM sodium lactate and 500 μM ISTD stocks into screw cap microcentrifuge tubes (Table 1). Lactate facilitates derivatization of the low concentration of 2HG found in the standards.
- Standards are vortexed and briefly centrifuged.
Table 1. Preparation of 2HG standards
- Standards are prepared in 80% methanol by combining 0-20 μl of the racemic 20 μM 2HG working solution with 2 μl each of the 10 mM sodium lactate and 500 μM ISTD stocks into screw cap microcentrifuge tubes (Table 1). Lactate facilitates derivatization of the low concentration of 2HG found in the standards.
- Derivatization
- Metabolite extracts (200 μl) are transferred to 2 ml screw cap microcentrifuge tubes.
- Standards and biological samples are evaporated to dryness using a Speedvac roto-evaporator with drying rate set to “HIGH” (~65 °C oven temperature). Complete evaporation of all water is critically important as the derivatization reaction will not occur in the presence of water. Evaporation may also be performed under nitrogen gas (Note 2).
- Derivatization reagent is prepared by dissolving DATAN in acetonitrile:acetic acid (4:1, v/v) to 50 mg/ml. The esterification reaction requires an aprotic solvent. Acetonitrile is more polar than dimethyl chloride, and we found this solvent more capable of dissolving the dried residue. Additionally, acetonitrile was directly compatible with our chromatographic approach.
- Dried samples are treated with 50 μl DATAN solution and heated at 70 °C for 2 h in a temperature controlled heat block. The derivatized standard solutions change color from clear to clear-yellow, while samples change from clear to dark yellow-brown. We previously used derivatization times as short as 30 min, but have found that 2 h results in consistently complete derivatization.
- Samples are cooled to room temperature and spun briefly.
- Derivatized samples are diluted with 50 μl acetonitrile:acetic acid (4:1, v/v), vortexed, spun briefly, and transferred to a microcentrifuge tube for LC-MS analysis.
Note: Diluting in acetonitrile:acetic acid was critically important for achieving adequate separation of the enantiomers chromatographically. Importantly, this method does not require an additional evaporation and resuspension step prior to LC-MS analysis as required by other published protocols.
- Metabolite extracts (200 μl) are transferred to 2 ml screw cap microcentrifuge tubes.
- Liquid chromatography
- Separation is performed on a ZIC-HILIC stationary phase with guard column.
- Mobile phases are prepared from 200 mM formic acid in LC-MS grade water titrated to pH 3.25 with ammonium hydroxide, acetonitrile, and LC-MS grade water.
- The autosampler wash solution is 95% LC-MS grade acetonitrile in LC-MS grade water.
- Samples are injected at 5 μl using a 20 μl sample injection loop.
- Liquid chromatography is performed using a 15% buffer A and 85% buffer B isocratic elution of 30 min per sample.
- Separation is performed on a ZIC-HILIC stationary phase with guard column.
- Mass spectrometry
- The mass spectrometer is tuned using a standard solution of racemic 2HG to optimize ionization and ion optics.
- The mass spectrometer is operated in selective reaction monitoring mode, and the following mass transitions are monitored over the duration of the chromatographic run: 363 > 147 + 129 m/z (derivatized 2HG), 147 > 129 m/z (2HG), and 149 > 105 m/z (ISTD). We found the signal intensity for the 147 > 129 m/z transition was much greater than the 363 > 147 m/z transition and used the former for quantification of 2HG enantiomers. We confirmed that underivatized and derivatized 2HG peaks were well separated chromatographically, and confirmed the retention times of the derivatized samples using the 363 > 147 m/z transition in standard samples (Figure 2).

Figure 2. Derivatization of 2HG standards. Standard solutions of D2HG (5 μM), L2HG (5 μM), and D2HG plus L2HG (5 μM each) were prepared at 5 μM in 200 μl of 80% methanol. These samples were evaporated, derivatized, and analyzed by LC-MS as described above. The 147 > 129 m/z extracted ion chromatogram of the racemic 2HG mixture (purple trace) demonstrated two well resolved peaks corresponding to the retention times observed for the derivatized D2HG sample (green trace) and the derivatized L2HG sample (blue trace). The green and blue traces are offset on the Y-axis by 5,000 intensity units for clarity. The 363 > 147 m/z extracted ion chromatogram (gray trace plotted on the right Y-axis) confirms these peaks represent derivatized 2HG. Underivatized 2HG elutes with a retention time of 6 min (not shown).
- The mass spectrometer is tuned using a standard solution of racemic 2HG to optimize ionization and ion optics.
- Data analysis
- Chromatographic peak areas for D2HG, L2HG, and the ISTD are integrated using Xcalibur software.
- Peak area ratios are calculated (D2HG/ISTD and L2HG/ISTD) for the standards to generate a standard curve (Figure 3).
- The sample concentration for each 2HG enantiomer is interpolated from the standard curve.
- Sample concentrations of 2HG should be normalized to some measure of sample mass (cell count, cell protein, tissue mass).
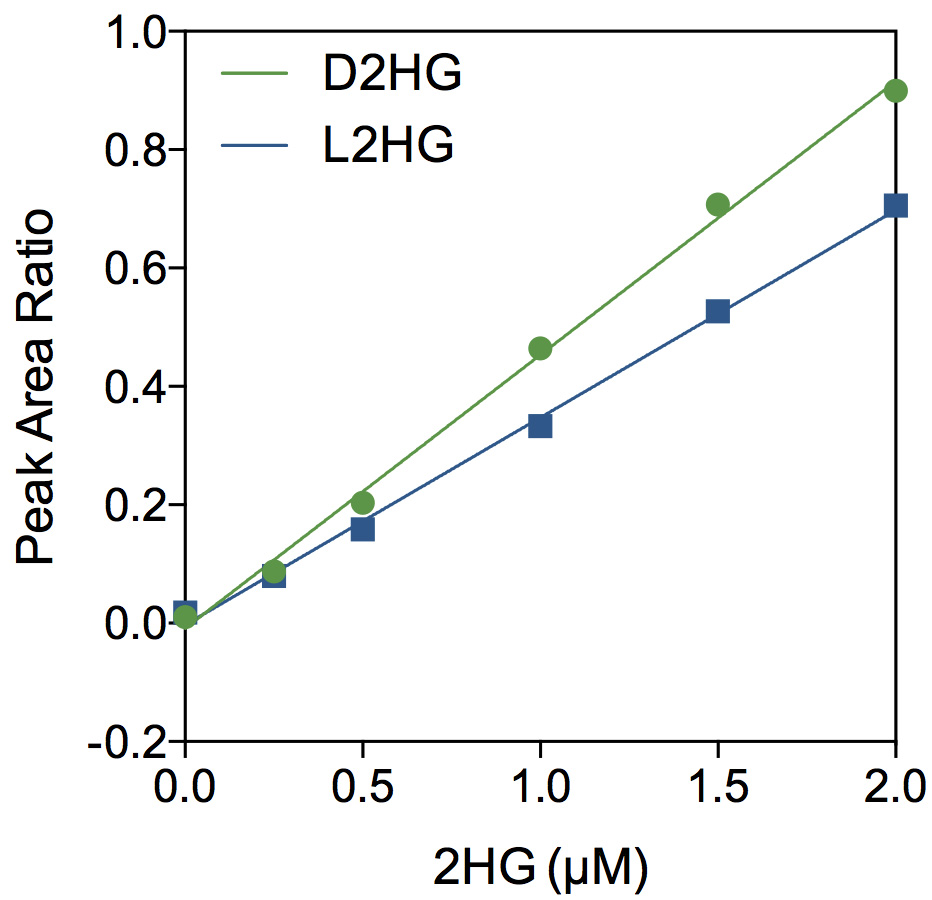
Figure 3. Representative standard curves
- Chromatographic peak areas for D2HG, L2HG, and the ISTD are integrated using Xcalibur software.
Representative data

Figure 4. A representative chromatogram from derivatized cellular extracts. Lung fibroblasts were treated with siRNA to silence expression of L2HGDH, the only enzyme known to metabolize L2HG. After 48 h, metabolites were extracted on dry ice using 80% methanol (Note 1). A 200 μl sample was evaporated and derivatized as described above. Peak areas were integrated using Xcalibur software and found to be: D2HG 967 arbitrary units (AU), L2HG 7,811 AU, and ISTD 65,470 AU (chromatogram not shown). Thus, the peak area ratios are: D2HG 0.015 and L2HG 0.119. The interpolated sample concentrations from the standard curve are: D2HG 51 nM and L2HG 348 nM. The mass of 2HG in the derivatized sample is determined by multiplying the concentration by the total volume, 100 μl. Therefore, the original sample contained 5.1 pmol D2HG and 34.8 pmol L2HG. We typically divide metabolite mass by the cell count for normalization.
Notes
- Biological samples from cells, tissue, plasma, and urine can be prepared using a variety of methods (Yuan et al., 2012). For our studies of cultured cells in 6-well dishes, we wash once with 2 ml of ice cold phosphate buffered saline per well and transfer the plates to a bed of dry ice. We then add 1 ml of 80% aqueous methanol pre-cooled to -80 °C and 10 μl of ISTD. The plates are then incubated at -80 °C for 15 min. The cells are then collected by scraping into a microcentrifuge tube. Insoluble material is pelleted by centrifugation at 15,000 x g at 4 °C. The supernatant is transferred to a new tube and aliquoted for LC-MS analysis.
- We found the roto-evaporator more useful as it concentrates the residue into a small pellet at the bottom of the tube while the nitrogen tended to disperse the dried residue throughout the tube.
- An alternative derivatization reagent, TSPC, has recently been reported to have improved sensitivity compared to DATAN derivatization (Cheng et al., 2015).
Recipes
- 50 mM D2HG and L2HG stock solution
Prepare 50 mM D2HG and L2HG stock solution in water
Aliquot and store at -20 °C until use - 20 μM 2HG working solution
- Dilute the 50 mM stocks to 1 mM
2 μl D2HG
2 μl L2HG
96 μl LC-MS grade water - Further dilute 1 mM 2HG to 20 μM in LC-MS grade water
- 10 mM sodium lactate stock solution
Prepare 10 mM in water
Aliquot and store at -20 °C until use - 500 μM internal standard (ISTD) stock solution
Prepare 500 μM [13C4]-2-oxoglutaric acid disodium salt stock solution in methanol
Store at -80 °C for use as an ISTD
Note: One may also chemically reduce (e.g., with zinc) isotopically labeled 2-oxoglutarate prior to derivatization for an isotopically labeled racemic 2HG internal standard. - Buffer A
10% 200 mM formic acid
90% LC-MS grade water - Buffer B
10% 200 mM formic acid
90% acetonitrile
Acknowledgments
This protocol was adapted from a prior publication (Struys, 2013). The work was supported by the NIH/NHLBI (HL007633 to WMO, and HL061795, HL108630, and GM107618 to JL) and the Brigham and Women’s Hospital Department of Medicine.
References
- Chalmers, R. A., Lawson, A. M., Watts, R. W., Tavill, A. S., Kamerling, J. P., Hey, E. and Ogilvie, D. (1980). D-2-hydroxyglutaric aciduria: case report and biochemical studies. J Inherit Metab Dis 3(1): 11-15.
- Cheng, Q. Y., Xiong, J., Huang, W., Ma, Q., Ci, W., Feng, Y. Q. and Yuan, B. F. (2015). Sensitive Determination of onco-metabolites of D- and L-2-hydroxyglutarate enantiomers by chiral derivatization combined with liquid chromatography/mass spectrometry analysis. Sci Rep 5: 15217.
- Cairns, R. A. and Mak, T. W. (2013). Oncogenic isocitrate dehydrogenase mutations: mechanisms, models, and clinical opportunities. Cancer Discov 3(7): 730-741.
- Duran, M., Kamerling, J. P., Bakker, H. D., van Gennip, A. H. and Wadman, S. K. (1980). L-2-Hydroxyglutaric aciduria: an inborn error of metabolism? J Inherit Metab Dis 3(4): 109-112.
- Dang, L., White, D. W., Gross, S., Bennett, B. D., Bittinger, M. A., Driggers, E. M., Fantin, V. R., Jang, H. G., Jin, S., Keenan, M. C., Marks, K. M., Prins, R. M., Ward, P. S., Yen, K. E., Liau, L. M., Rabinowitz, J. D., Cantley, L. C., Thompson, C. B., Vander Heiden, M. G. and Su, S. M. (2009). Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 462(7274): 739-744.
- Intlekofer, A. M., Dematteo, R. G., Venneti, S., Finley, L. W., Lu, C., Judkins, A. R., Rustenburg, A. S., Grinaway, P. B., Chodera, J. D., Cross, J. R. and Thompson, C. B. (2015). Hypoxia iduces production of L-2-hydroxyglutarate. Cell Metab 22(2): 304-311.
- Kranendijk, M., Struys, E. A., Salomons, G. S., Van der Knaap, M. S. and Jakobs, C. (2012). Progress in understanding 2-hydroxyglutaric acidurias. J Inherit Metab Dis 35(4): 571-587.
- Mullen, A. R., Hu, Z., Shi, X., Jiang, L., Boroughs, L. K., Kovacs, Z., Boriack, R., Rakheja, D., Sullivan, L. B., Linehan, W. M., Chandel, N. S. and DeBerardinis, R. J. (2014). Oxidation of alpha-ketoglutarate is required for reductive carboxylation in cancer cells with mitochondrial defects. Cell Rep 7(5): 1679-1690.
- Oldham, W. M., Clish, C. B., Yang, Y. and Loscalzo, J. (2015). Hypoxia-mediated increases in L-2-hydroxyglutarate coordinate the metabolic response to reductive stress. Cell Metab 22(2): 291-303.
- Reinecke, C. J., Koekemoer, G., van der Westhuizen, F. H., Louw, R., Lindeque, J. Z., Mienie, L. J., Smuts, I. (2012). Metabolomics of urinary organic acids in respiratory chain deficiencies in children. Metabolomics 8(2): 264-283.
- Struys, E. A. (2013). 2-Hydroxyglutarate is not a metabolite; D-2-hydroxyglutarate and L-2-hydroxyglutarate are!. Proc Natl Acad Sci U S A110(51): E4939.
- Struys, E. A., Jansen, E. E., Verhoeven, N. M. and Jakobs, C. (2004). Measurement of urinary D- and L-2-hydroxyglutarate enantiomers by stable-isotope-dilution liquid chromatography-tandem mass spectrometry after derivatization with diacetyl-L-tartaric anhydride. Clin Chem 50(8): 1391-1395.
- Ward, P. S., Patel, J., Wise, D. R., Abdel-Wahab, O., Bennett, B. D., Coller, H. A., Cross, J. R., Fantin, V. R., Hedvat, C. V., Perl, A. E., Rabinowitz, J. D., Carroll, M., Su, S. M., Sharp, K. A., Levine, R. L. and Thompson, C. B. (2010). The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell 17(3): 225-234.
- Yuan, M., Breitkopf, S. B., Yang, X. and Asara, J. M. (2012). A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat Protoc 7(5): 872-881.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Oldham, W. M. and Loscalzo, J. (2016). Quantification of 2-Hydroxyglutarate Enantiomers by Liquid Chromatography-mass Spectrometry. Bio-protocol 6(16): e1908. DOI: 10.21769/BioProtoc.1908.
Category
Biochemistry > Other compound > Acid
Cancer Biology > Cellular energetics > Biochemical assays > Other compound
Cell Biology > Cell metabolism > Amino acid
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link












