Innate lymphoid cells (ILCs) are a rare cell population subdivided into ILC1s, ILC2s, and ILC3s, based on transcription factor expression and cytokine production. In models of lung inflammation, the release of alarmins from the epithelium activates ILC2s and promotes the production of Th2-cytokines and the proliferation and migration of ILC2s within the lung. ILC2s are the innate counterpart to CD4+ Th2s and, as such, express Gata-3 and produce IL-4, IL-5, and IL-13. Due to the low number of ILCs and the lack of specific surface markers, flow cytometry is the most reliable technique for the identification and characterization of ILCs. In this protocol, multicolor flow cytometry is utilized to identify Lineage−Thy1.2+ ILCs. Intracellular cytokine staining further identifies ILC2s within the lung. This protocol presents a reliable method for promoting ILC2-mediated lung inflammation and for monitoring ILC2 biology.
Key features
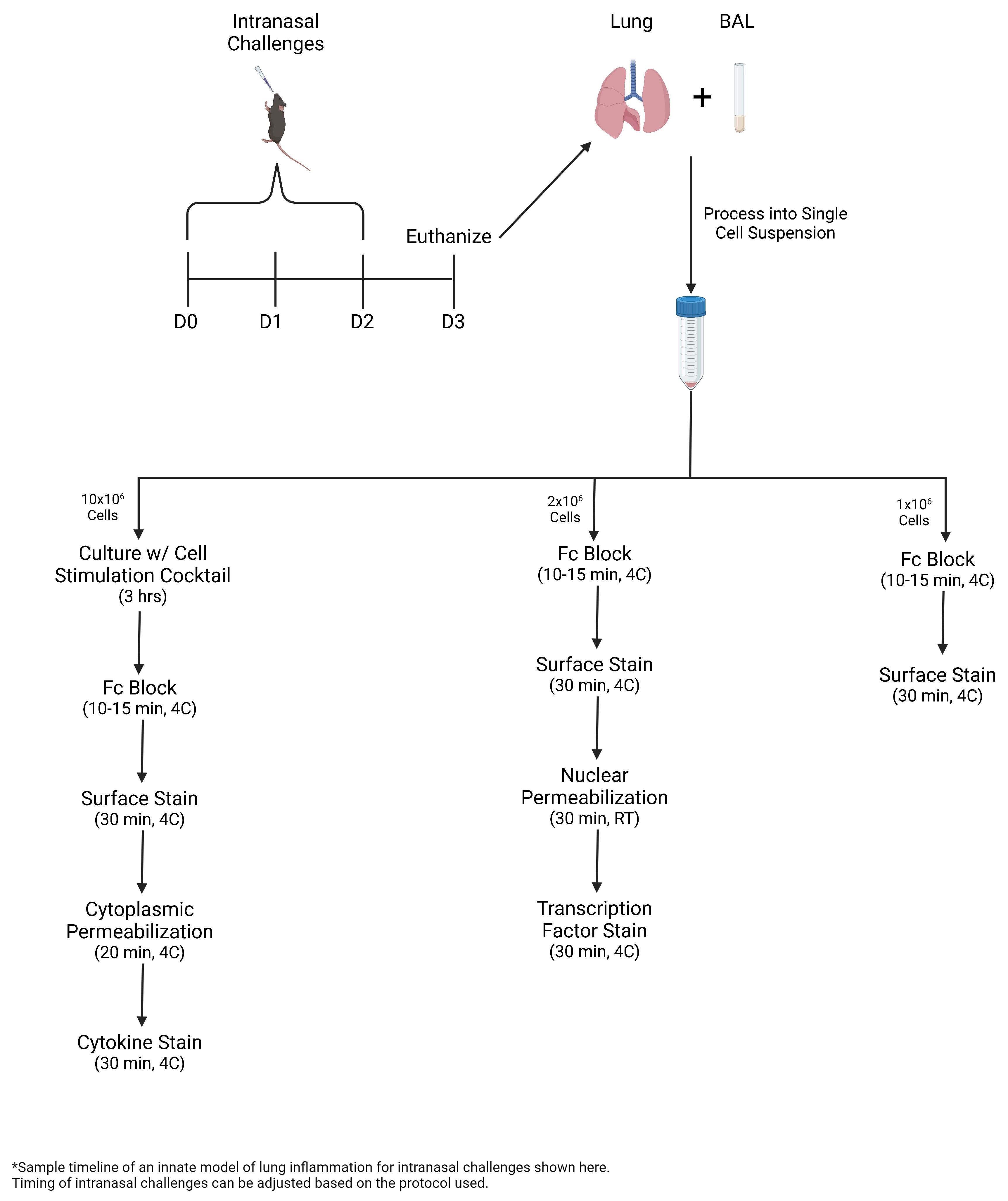
• In this protocol, ILC2s are expanded via intranasal challenges with Alternaria alternata, a fungal allergen, or recombinant IL-33.
• Bronchoalveolar lavage (BAL) and lung are collected and processed into single-cell suspension for multicolor flow cytometric analysis, including intracellular staining of transcription factors and cytokines.
• During lung inflammation, the percentage of ILC2s and eosinophils increases. ILC2s express greater levels of Gata-3 and Ki-67 and produce greater amounts of IL-5 and IL-13.
Graphical overview